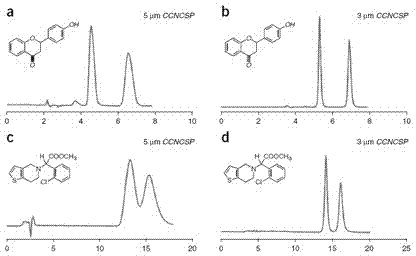
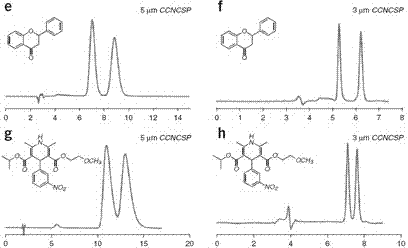
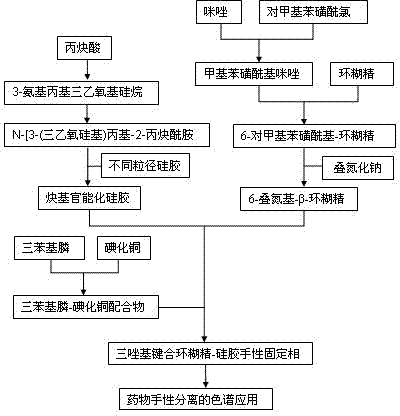
Triazolyl bonded cyclodextrin-silica gel chiral stationary phase and preparation method thereof
A technology of chiral stationary phase and cyclodextrin, which is applied in chemical instruments and methods, other chemical processes, etc., can solve the problems of limited types of chiral resolution drugs, chiral stationary phase bonding instability, and chiral resolution The ability needs to be improved, etc., to achieve excellent acid-base stability, maintain chemical stability, and deterministic structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Taking β-cyclodextrin as an example
[0038] In conjunction with the accompanying drawings, the preparation method of the double substituted double punctual center-β-cyclodextrin of the present invention comprises the following steps:
[0039] In the first step, p-toluenesulfonyl chloride and imidazole are reacted at room temperature in dichloromethane to obtain p-toluenesulfonyl imidazole;
[0040] In the second step, the product p-toluenesulfonyl imidazole obtained in the first step is placed in the aqueous solution of dissolving β-cyclodextrin, stirred at room temperature for 2~4 h, and then sodium hydroxide solution is added, and a small amount of precipitate produced is removed by filtration Add ammonium chloride to the filtrate to adjust its pH value to 6-9 to obtain a white solid substance, filter to obtain the product p-toluenesulfonyl-β-cyclodextrin (Ts-CD), and vacuum-dry the product;
[0041] In the third step, take a double-necked round bottom fl...
Embodiment 2
[0066] Example 2 with α-cyclodextrin as an example
[0067] The method for preparing a triazolyl-bonded cyclodextrin-silica gel chiral stationary phase with broad-spectrum chiral resolution capability is characterized in that it comprises the following steps:
[0068] The first step, based on the mechanism of nucleophilic substitution, 1 mol of p-toluenesulfonyl chloride and 3 mol of imidazole in dichloromethane, 25 o C reaction obtains p-methylbenzenesulfonyl imidazole overnight;
[0069] In the second step, 2 mol of p-toluenesulfonyl imidazole, the product obtained in the first step, is placed in an aqueous solution of 1 mol α-cyclodextrin, 25 o After C stirred and reacted for 4 h, add mass percent and be 30% sodium hydroxide aqueous solution, filter; Add ammonium chloride to the filtrate to adjust its pH value to 8 to obtain a white solid substance, filter to obtain the product p-toluenesulfonyl-α - Cyclodextrin Ts-CD, vacuum dried product;
[0070] In the third step, ...
Embodiment 3
[0076] The method for preparing a triazolyl-bonded cyclodextrin-silica gel chiral stationary phase with broad-spectrum chiral resolution capability is characterized in that it comprises the following steps:
[0077] The first step, based on the mechanism of nucleophilic substitution, 1 mol of p-toluenesulfonyl chloride and 3 mol of imidazole in dichloromethane, 25 o C reaction obtains p-methylbenzenesulfonyl imidazole overnight;
[0078] In the second step, 2 mol of p-toluenesulfonyl imidazole, the product obtained in the first step, is placed in an aqueous solution of 1 mol of γ-cyclodextrin, 25 o After stirring the reaction for 4 h, add mass percent and be 30% sodium hydroxide aqueous solution, filter; add ammonium chloride to the filtrate to adjust its pH value to 8 to obtain a white solid substance, and filter to obtain the product p-toluenesulfonyl-γ - Cyclodextrin Ts-CD, vacuum dried product;
[0079] In the third step, 6-p-methylbenzenesulfonyl-γ-cyclodextrin (3.1 g, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com