Ceforanide C-type crystal composition used for injection and its preparation method
A technology for cefret and injection, which is applied in the field of cefret C-type crystalline composition for injection and preparation thereof, and achieves the effects of being conducive to the protection of the natural environment, high purity and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
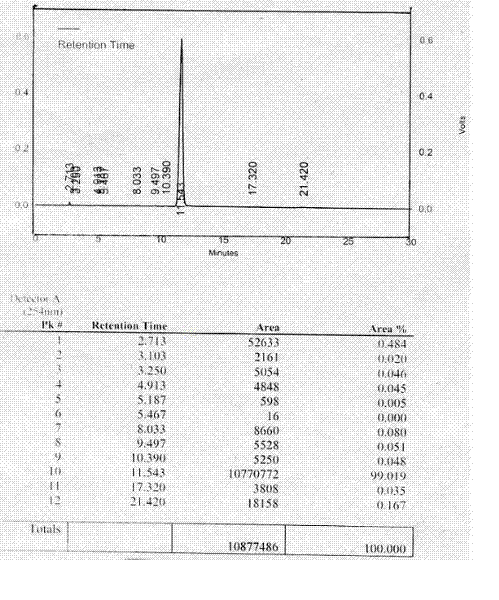
[0049] Embodiment 1: Preparation of Cefereide Type A crystals:
[0050] According to the patent application number 200910223572.5 in the example 9 obtained cefereide N,N-dimethylbenzylamine salt (CRD-H DMBA) compound [2] 65.48g, placed in a 1000ml three-necked bottle, add 328ml Deionized water (w / v=1:5), stir to dissolve, keep warm in a water bath (30°C), when most of the solids are dissolved, add 3.0g NaHCO 3 Stirring, vacuum extraction of CO 2 Gas to make it completely dissolved, measure the pH value of the solution, and adjust the solution to PH=7.5±0.2 with a little 2N hydrochloric acid. Then decolorize with 6.5g of activated carbon in vacuum pumping, filter the solution through water-soluble filter membranes with pore diameters of 0.45μm and 0.22μm respectively, then wash twice with 100ml of deionized water, combine the washings to obtain crystallization solution; Internally, use 2N hydrochloric acid to adjust the pH value of the isoelectric point to crystallize, that i...
Embodiment 2
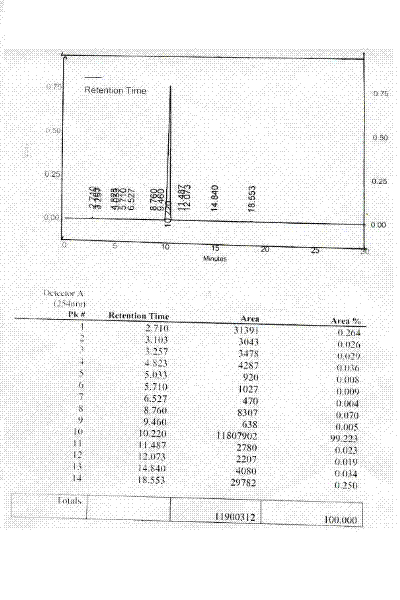
[0054] Embodiment 2: Preparation of L-lysine type B crystals:
[0055] Dissolve 25.0 g of commercially available spray-dried L-lysine (alkali) in 125 ml of deionized water, add 5.0 g of activated carbon, and stir at room temperature for 60 minutes for decolorization. Sterile filtration, washing and filtering twice with 25ml deionized water and combining the filtrate washings, and placing them in a constant pressure dropping funnel for later use.
[0056] Treat with activated carbon, filter aseptically, place 3000ml of filtered isopropanol in a 1 liter three-neck bottle and seal it, drop the above L-lysine solution into the isopropanol at a constant speed of 40 drops per minute After the dropwise addition, stir at room temperature to make it precipitate and turn into crystals. When the process is observed with a polarizing microscope, it can be seen that the product is in a state of solution from hygroscopicity to non-hygroscopic under microscope inspection, and crystals are ob...
Embodiment 3
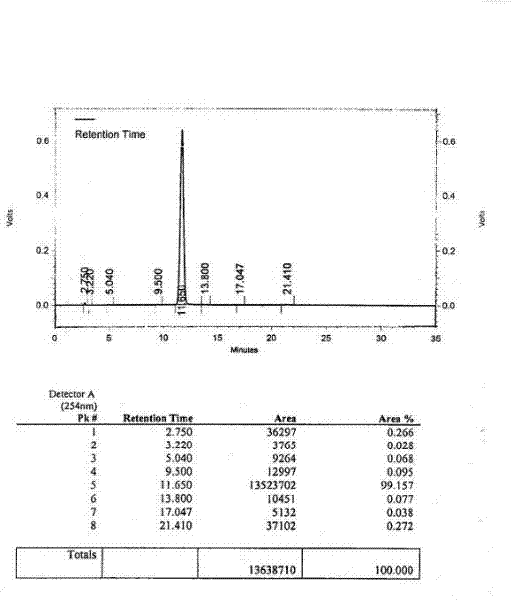
[0058] Embodiment 3: Preparation of cefereide type C crystalline composition for injection:
[0059] The cefereide type A crystals and L-lysine type B crystals obtained in Example 1 and Example 2 were respectively pulverized with a pulverizer, passed through a 180-200 mesh sieve, and set aside.
[0060] Mix according to the molar ratio of cefereide type A crystal and L-lysine type B crystal at 1:1~1:1.2, fully mix in the three-dimensional powder mixer, and then pack in 1.3g-1.32g per bottle molded glass bottle, that is, the target object.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com