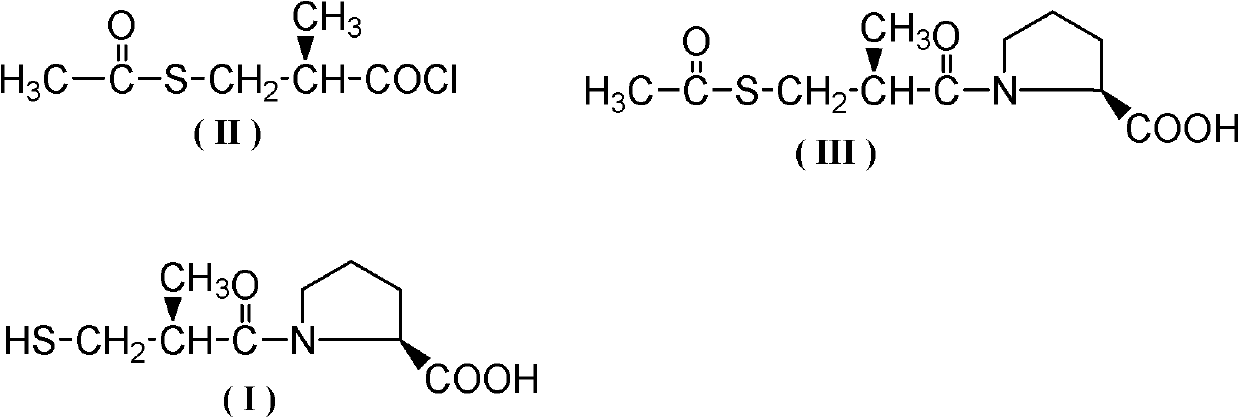
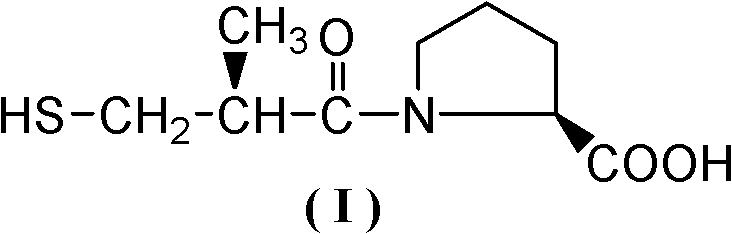
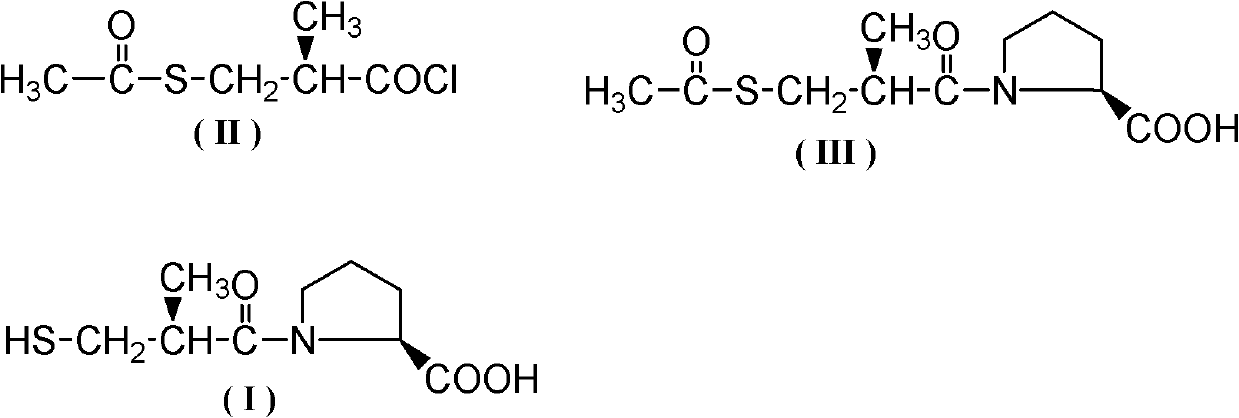
Method for synthesizing captopril and ceptopril
A synthesis method and technology of propionyl chloride are applied in the synthesis field of captopril and acyltopril, and can solve the problems of many steps of the splitting method, low product yield and high production cost, and achieve low production cost and high reaction yield. The effect of high and three wastes is small
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]Add 200g (1.23mol) D-3-acetylthio-2-methyl-propionic acid, 110g (0.37mol) bis(trichloromethyl)carbonic acid to a 500mL three-necked flask equipped with mechanical stirring, a thermometer and a drying tube Esters and 2g (0.023mmol) N,N-dimethylacetamide, stirred and heated to 30°C for 50h, after the reaction, control the vacuum degree to 5mmHg, first remove the low boilers at 90-100°C by vacuum distillation, The fractions at 110-115°C were collected again to obtain 199.5 g of colorless liquid D-3-acetylthio-2-methyl-propionyl chloride, with a yield of 89.5% and a content detected by HPLC of 97.8%.
Embodiment 2
[0036] Add 200g (1.23mol) D-3-acetylthio-2-methyl-propionic acid, 183g (0.62mol) bis(trichloromethyl)carbonic acid to a 500mL three-necked flask equipped with mechanical stirring, a thermometer and a drying tube Ester and 0.1g (0.0014mol) N,N-dimethylformamide, stirred and heated to 60°C for 35h. After the reaction, control the vacuum degree to 5mmHg, first remove the low boilers at 90-100°C by vacuum distillation, and then collect the fraction at 110-115°C to obtain colorless liquid D-3-acetylthio-2-methyl-propane Acid chloride 188.5g, yield 84.6%, HPLC detection content 98.1%.
Embodiment 3
[0038] Add 200g (1.23mol) D-3-acetylthio-2-methyl-propionic acid, 129g (0.43mol) bis(trichloromethyl)carbonic acid to a 500mL three-necked flask equipped with mechanical stirring, a thermometer and a drying tube Ester and 9g (0.056mol) N, N-carbonyldiimidazole, stirred and heated to 70°C for 25h. After the reaction, control the vacuum degree to 5mmHg, first remove the low boilers at 90-100°C by vacuum distillation, and then collect the fraction at 110-115°C to obtain colorless liquid D-3-acetylthio-2-methyl-propane Acid chloride 193.6g, yield 86.9%, HPLC detection content 97.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com