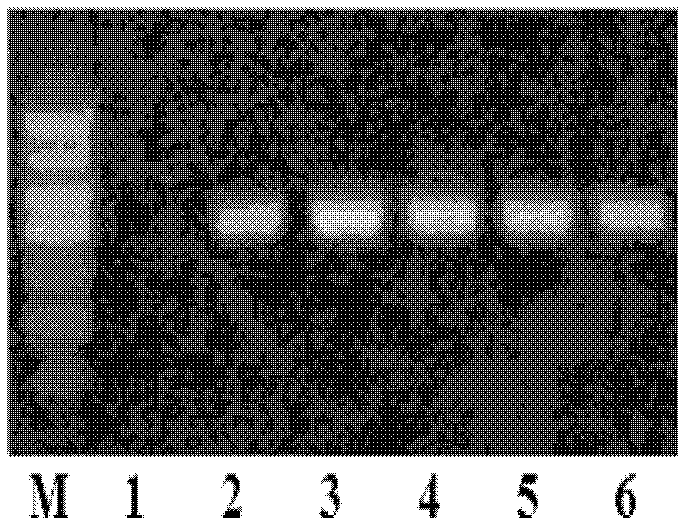Fusion protein capable of inhibiting tumor neovascularization and production technology thereof
A technology for tumor neovascularization and fusion proteins, which is applied in the field of long-acting recombinant proteins, can solve the problems of limiting therapeutic effects, and achieve the effects of convenient downstream purification, process-based production, and high levels of secreted proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] 1. Construction of expression vector
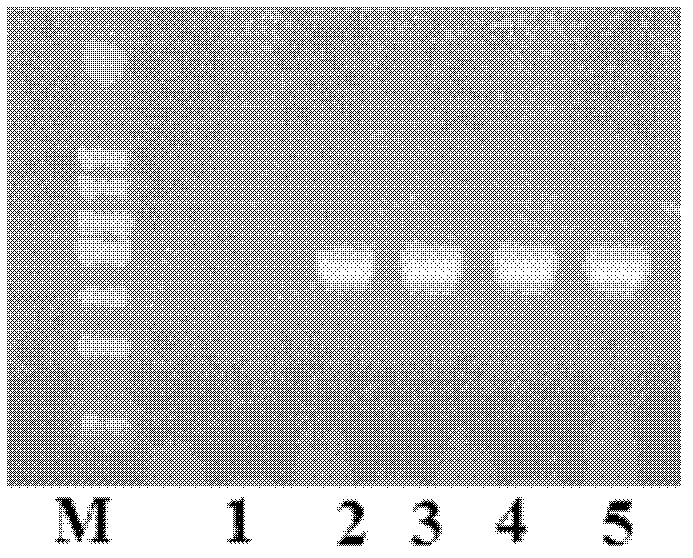
[0031] According to the NCBI reference sequence of human Kal cDNA (Reference Sequence: NM_006215.2) and the NCBI reference sequence of hCGβ chain (Reference Sequence: NM_033378.1), we commissioned Dalian Bao Biological Company to fully synthesize the fusion sequence of human Kal cDNA and hCGβ-CTP ( figure 1 , see the sequence listing SEQ ID No.1). hCGβ-CTP is the 118th-145th amino acid sequence of the C-terminus of hCGβ chain. Hind III and EcoR I restriction sites were introduced at the 5' and 3' ends of the fusion sequence, respectively, in order to construct eukaryotic expression vectors.
[0032] The fusion sequence containing the full-length Kal cDNA sequence and the hCGβ-CTP sequence was subjected to HindIII and EcoRI double digestion, and connected to the HindIII and EcoRI double restriction site of the pcDNA3.1 plasmid produced by Invitrogen to obtain pcDNA3.1-LA- Kal(28) expression plasmid.
[0033] The pcDNA3.1-LA-Kal(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com