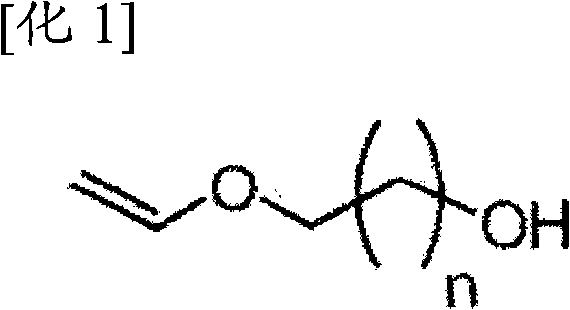
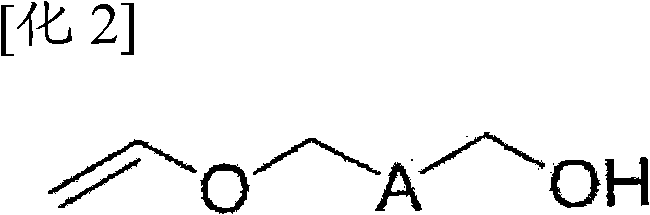
Method for preparing (methyl) crylic acid hydroxyalkyl ester
A technology of hydroxyalkyl acrylate and manufacturing method, which is applied in the direction of reaction preparation of ester group and hydroxyl group, preparation of carboxylate, chemical instruments and methods, etc., can solve problems such as easy gelation, and achieve high-purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1
[0058] (Synthesis of Vinyloxybutyl Methacrylate)
[0059] Add 1000 g of 4-hydroxybutyl vinyl ether (manufactured by Maruzen Petrochemical Co., Ltd. HBVE), 3000 g of methyl methacrylate, and 0.65 g of p-methoxyphenol into a 4 L four-necked round-bottom detachable flask, and install a rectification column ( 15 segments), mixer, air inlet tube, thermometer. Under stirring, start heating while introducing dry air at a rate of 100ml / min, adjust the pressure to about 40kPa so that the liquid temperature in the flask is 75°C during reflux, and remove the moisture in the system. After confirming that the water content in the system was 300 ppm or less, 8.6 g of titanium tetraisopropoxide was added as a catalyst, and the pressure in the flask was controlled to about 60 kPa so that the reaction temperature was 95±5°C. When heating to reflux, monitor the temperature of the upper part of the rectification column (column top temperature). Since it is close to the azeotropic temperature of...
Embodiment 1
[0061] (Synthesis of 4-hydroxybutyl methacrylate using ethylene glycol)
[0062] 2.5 g of p-toluenesulfonic acid and 101 g of ethylene glycol were charged into a 1 L four-necked separable flask, and a stirrer, a thermometer, an air introduction tube, and a vacuum pump with a cold trap were installed. While stirring, 250 g of vinyloxybutyl methacrylate synthesized in Production Example 1 was gradually added, and the liquid temperature was adjusted to maintain at 40°C. After the addition, the pressure was reduced to 30 kPa, and the stirring was continued for 1 hour while introducing dry air at a rate of 100 ml / min. Then, the reaction liquid was analyzed by gas chromatography, and no vinyloxybutyl methacrylate peak was found. However, in the analysis by liquid chromatography, it was confirmed that 14.4% of acetal dimers were produced, so 25.2 g of ethylene glycol was added and the pressure was set at 5 kPa to perform deacetalization reaction. Analysis was carried out after 2 hou...
Embodiment 2
[0064] (Synthesis of 4-hydroxybutyl methacrylate using propylene glycol)
[0065] 2.5 g of p-toluenesulfonic acid and 124 g of propylene glycol were charged into a 1 L four-necked detachable flask, and a stirrer, a thermometer, an air inlet tube, and a vacuum pump with a cooling trap were installed. While stirring, 250 g of vinyloxybutyl methacrylate synthesized in Production Example 1 was gradually added, and the liquid temperature was adjusted to maintain at 40°C. After the addition, the pressure was reduced to 30 kPa, and the stirring was continued for 1 hour while introducing dry air at a rate of 100 ml / min. Then, the reaction liquid was analyzed by gas chromatography, and no vinyloxybutyl methacrylate peak was found. However, in the analysis by liquid chromatography, it was confirmed that 16.2% of acetal dimers were produced, so 31 g of propylene glycol was added and the deacetalization reaction was carried out at a pressure of 5 kPa. Analysis was carried out after 2 hou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com