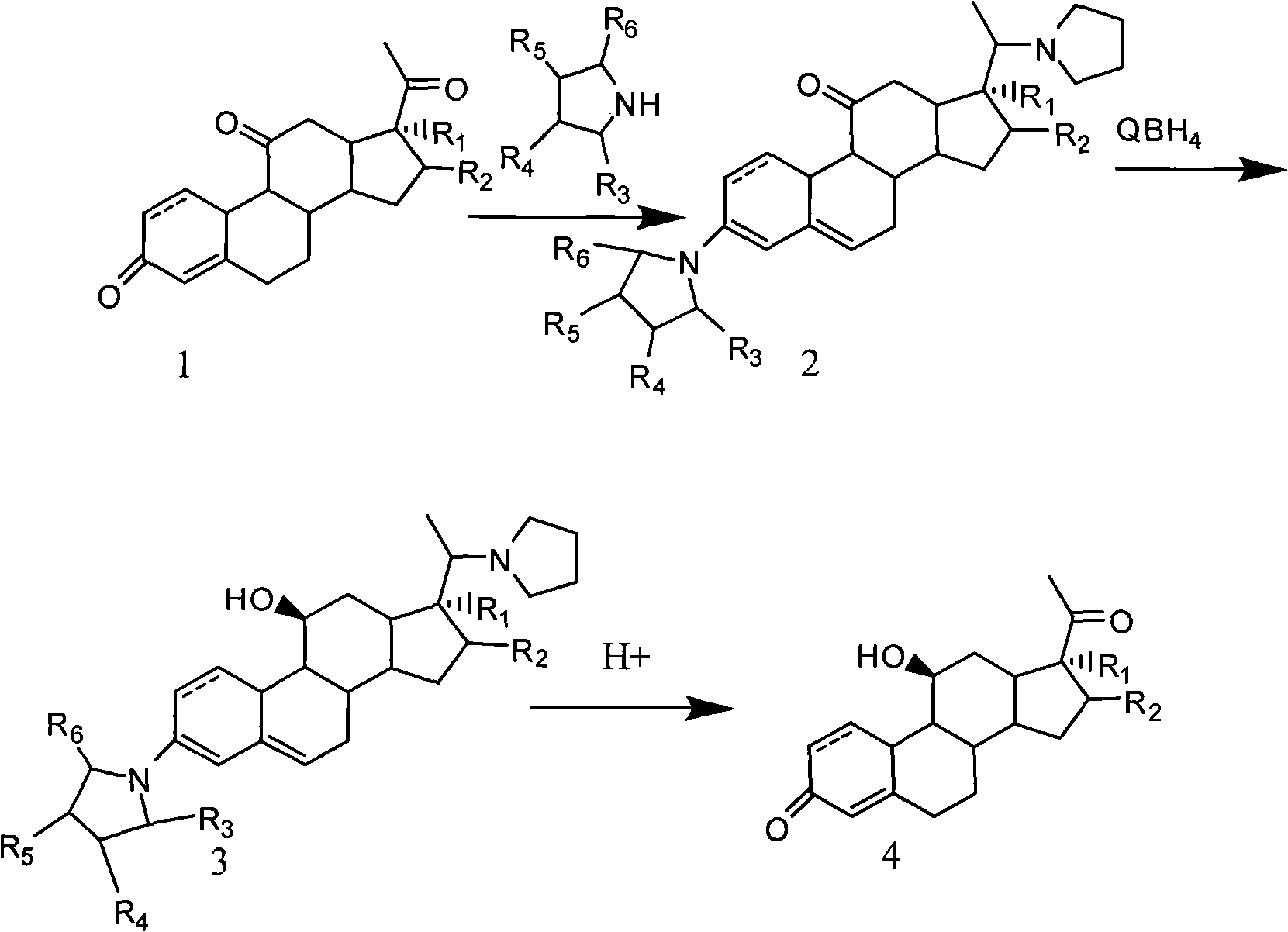
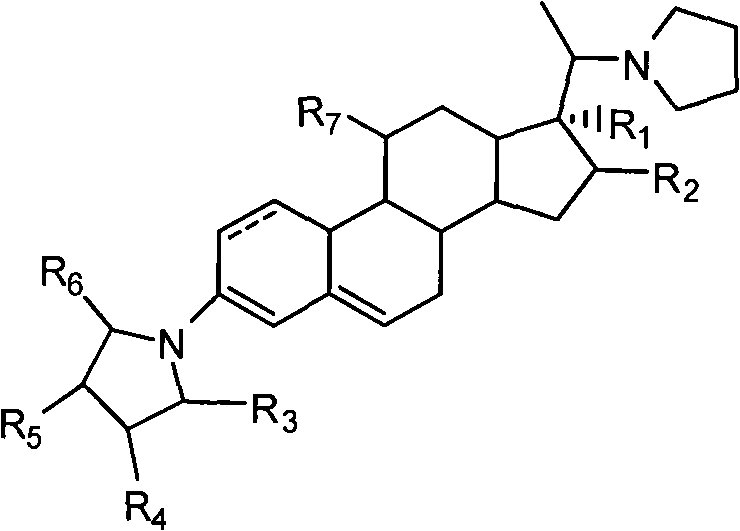
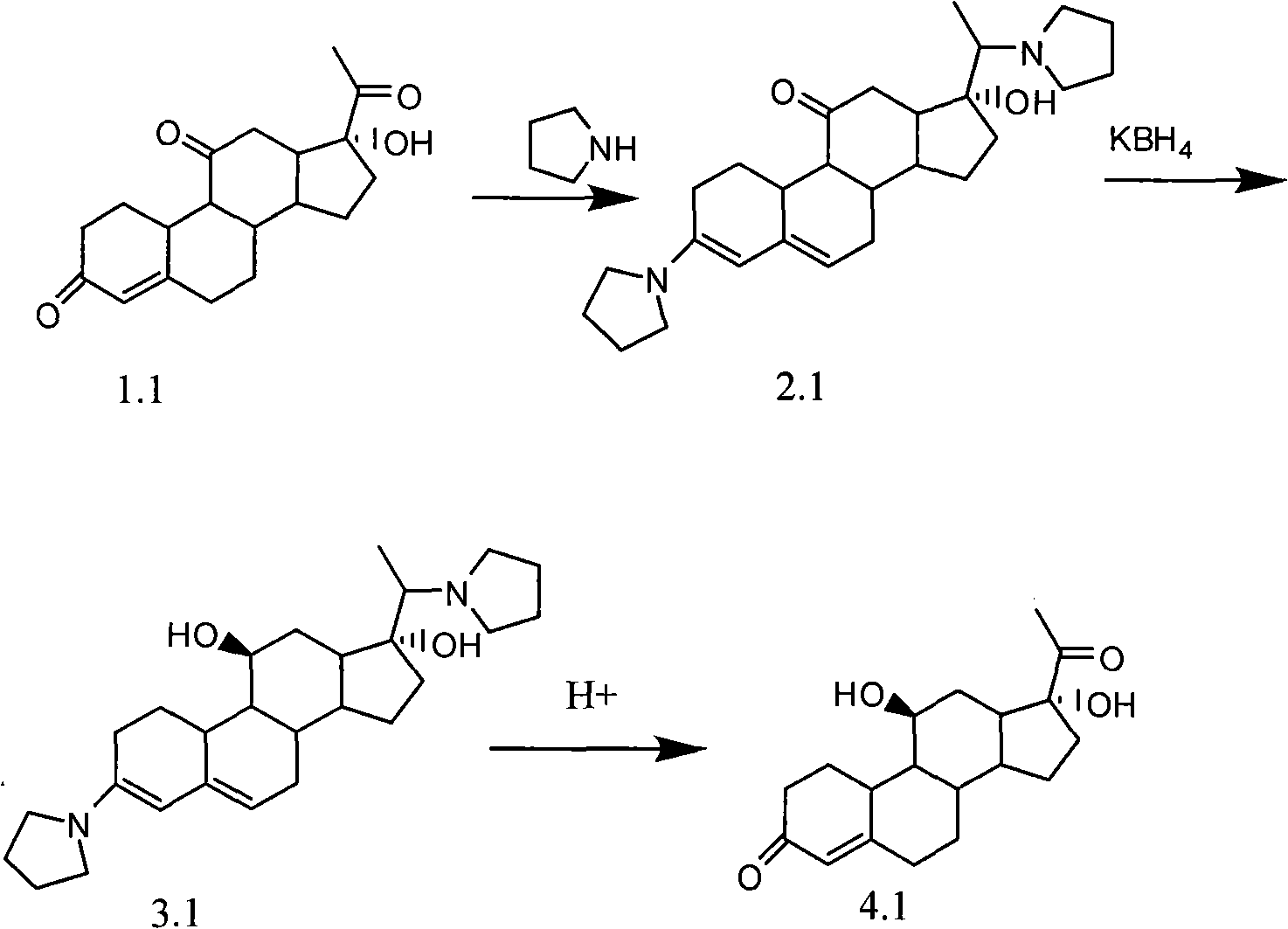
Novel technology for synthesizing pregnene 11-site beta-hydroxy
A compound and reaction technology, applied in the field of production technology for preparing prednisolone and derivatives thereof, can solve the problems of incomplete hydrolysis, great difficulty in deprotection, easy generation of impurities, etc., so as to reduce labor costs and reduce solvent consumption. , the effect of simplifying the operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
[0036] step a
[0037] Add 10mmol of compound 1.1 into 50ml of ethanol, reflux and add 45mmol of tetrahydropyrrole and 0.6ml of triethyl orthoformate under nitrogen protection, react until there is no raw material, concentrate under reduced pressure and cool to 0°C, dilute in 100ml of ice water, filter , to obtain 2.19.4 mmol of solid.
[0038] step b
[0039] Add 2.19mmol of the solid to 50ml of methanol and 2ml of pyridine, stir and heat to 40-45°C, add KBH within 15 minutes 4 12mmol, react until there is no raw material, control the temperature below 40°C and add acetic acid dropwise until the pH is 7, concentrate under reduced pressure and cool down to 0°C, dilute in 100ml ice water, filter to obtain 3.18.2mmol of solid.
[0040] step c
[0041] Add 3.15mmol of the solid to 50ml of 10% acetic acid aqueous solution, stir at room temperature, and react until no raw material is found. After extracting three times with 30ml of chloroform, check the concentration...
Embodiment 2
[0043]
[0044] step a
[0045] Add 10mmol of compound 1.2 into 50ml of cyclohexane, reflux and separate water, add 50mmol of 2-methyltetrahydropyrrole dropwise under nitrogen protection, react until no raw materials are left, concentrate under reduced pressure and cool to 0°C, precipitate solid 2.2, filter , to obtain 2.29.0 mmol of solid.
[0046] step b
[0047] Add 2.28mmol of the solid into 50ml of methanol, stir and heat to 40-45°C, add NaBH within 15 minutes 4 12mmol, react until there is no raw material, control the temperature below 40°C and gradually add acetic acid until the pH is 7, concentrate under reduced pressure and cool down to 0°C, dilute in 100ml ice water, filter to obtain 3.27.1mmol of solid.
[0048] step c
[0049] Add 3.25mmol of the solid to 50ml of 10% aqueous acetic acid solution, stir at room temperature, and react until there is no raw material. After extracting three times with 30ml of chloroform, check the concentration, recrystallize with...
Embodiment 3
[0051]
[0052] step a
[0053] Add 10mmol of compound 1.3 into 50ml of absolute ethanol, reflux and add 50mmol of tetrahydropyrrole and 0.6ml of triethyl orthoformate under nitrogen protection, react until there is no raw material, concentrate under reduced pressure and cool to 0°C, dilute in 100ml of ice water , filtered to obtain 2.18.9 mmol of solid.
[0054] steps b and c
[0055]Add 2.18mmol of the solid to 50ml of methanol and 2ml of pyridine, stir and heat to 40-45°C, then add 0.03g of CdCl 2 (2 / 5)H 2 O, join KBH in 15 minutes 4 12mmol, react until there is no raw material, control the temperature below 40°C and add 50% acetic acid aqueous solution dropwise to pH 3, concentrate under reduced pressure and cool to 0°C, dilute in 100ml ice water, filter to obtain 4.37.1mmol of solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com