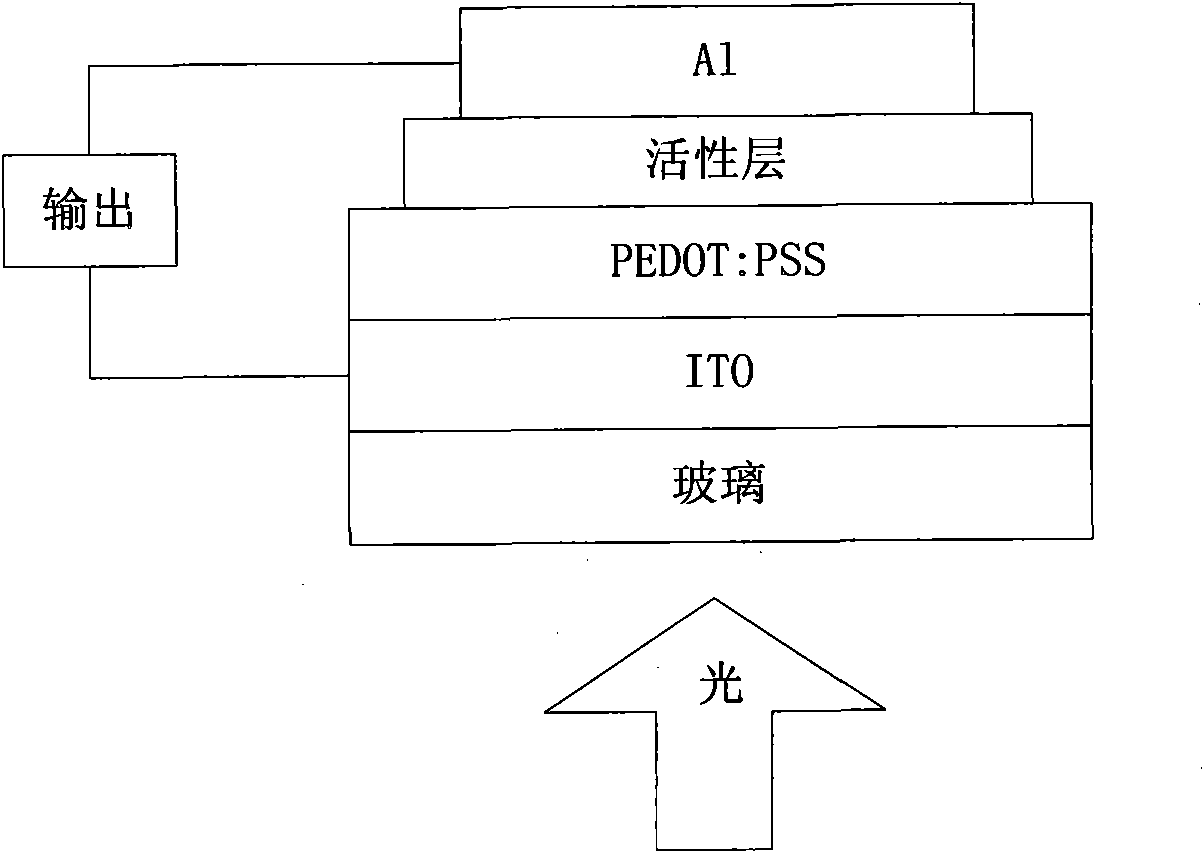
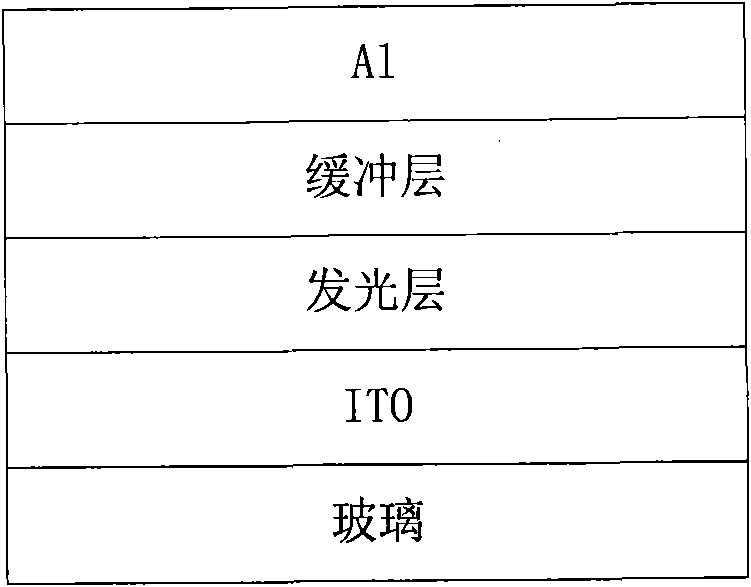
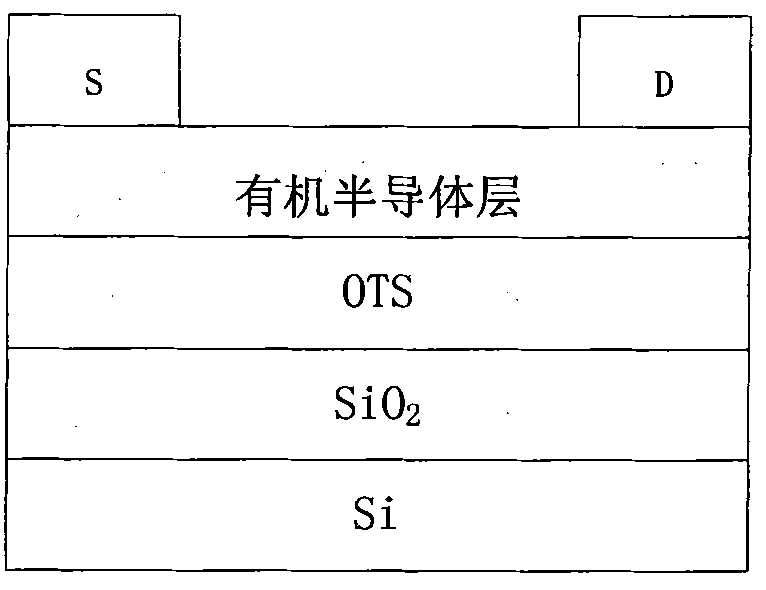
Perylenetetracarboxylic diimide copolymers, preparation method thereof and application thereof
A perylene tetracarboxylic acid diimide copolymer and a technology of perylene tetracarboxylic acid diimide, which are applied in the field of copolymers, can solve the problem of reducing the photoelectric conversion efficiency of organic solar cells, failing to effectively utilize sunlight, and matching emission spectra. Insufficient degree of light and other problems, to achieve the effect of excellent charge transport performance, wide range of light absorption, and excellent solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Poly N,N'-bis-(3,4,5-tri-methylbenzene)-3,4,9,10-perylenediimide-1,1,3,4-tetramethyl-2, 5-bisphenylsilole (n=9):
[0037]
[0038] Under the protection of nitrogen, to the compound N,N'-bis-(3,4,5-tri-methylbenzene)-1,7-dibromo-3,4,9,10-perylene diimide 0.5 A solution of 0.5 mmol of 1,1,3,4-tetramethyl-2,5-bis(4-tributyltin-phenyl)silole in N,N-dimethylformamide (18 mL). Nitrogen was introduced, and the residual oxygen was removed by bubbling for 0.5h. Then join Pd 2 (dba) 3 (0.014g, 0.015mmol) and P(o-Tol) 3 (0.0083g, 0.027mmol); continue to feed nitrogen, bubbling for 0.5h to remove residual oxygen, and then heated to 80°C for 48 hours to obtain poly N,N'-bis-(3,4,5-tri- Reaction mixture of methylbenzene)-3,4,9,10-perylenediimide-1,1,3,4-tetramethyl-2,5-bisphenylsilole (n=9) .
[0039] Add the reactant mixture dropwise to methanol for sedimentation, filter with suction, wash with methanol, and dry; then dissolve in toluene, add to the aqueous solution of sod...
Embodiment 2
[0041] Poly N,N'-bis-(3,5-dimethyloxy-4-dodecyloxybenzene)-3,4,9,10-perylenediimide-1,1-dioctyl- 3,4-diphenyl-2,5-bisphenylsilole (n=23):
[0042]
[0043] Under the protection of argon, to the compound N,N'-bis-(3,5-dimethyloxy-4-dodecyloxy)-1,7-dibromo-3,4,9,10- Perylene diimide 0.5mmol, 1,1-dioctyl-3,4-diphenyl-2,5-bis(4-tributyltin-phenyl)silole 0.5mmol of dioxane (15mL) solution. Introduce argon, bubble 0.5h to remove residual oxygen. Then add Pd(PPh 3 ) 2 Cl 2 10mg, continued to pass through argon, bubbled for 0.5h to remove residual oxygen, and then heated to 85 ° C for 36 hours to prepare poly N, N'-bis-(3,5-dimethyloxy-4-deca Dialkoxyphenyl)-3,4,9,10-perylenediimide-1,1-dioctyl-3,4-diphenyl-2,5-bisphenylsilole (n=23 ) reactant mixture.
[0044] The mixture was added dropwise to methanol for settling. Suction filtration, washing with methanol, and drying; then dissolved in toluene, added to the aqueous solution of sodium diethyldithiocarbamate; then the mi...
Embodiment 3
[0046] Poly N,N'-di-(3,4,5-tri-eicosyloxybenzene)-3,4,9,10-perylene diimide-1,1,3,4-tetra-di Decyl-2,5-bisphenylsilole (n=100):
[0047]
[0048] Under the protection of mixed gas of nitrogen and argon, to the compound N, N'-bis-(3,4,5-tri-eicosyloxybenzene)-1,7-dibromo-3,4,9, 0.5 mmol of 10-perylene diimide, 0.5 mmol of 1,1,3,4-tetra-eicosyl-2,5-bis(4-tributyltin-phenyl)silole 0.5 mmol in toluene / THF (30 mL) solution. A mixture of nitrogen and argon was introduced, and the residual oxygen was removed by bubbling for 0.5h. Then add Pd(PPh 3 ) 4 8mg, continue to pass through the mixed gas of nitrogen and argon, bubble 0.5h to remove the residual oxygen, and then heat to 80°C for 72 hours to prepare poly N,N'-bis-(3,4,5-tri-di Decyloxyphenyl)-3,4,9,10-perylenediimide-1,1,3,4-tetra-eicosyl-2,5-bisphenylsilole (n=100) reactant mixture.
[0049] The mixture was added dropwise to methanol for sedimentation, suction filtered, washed with methanol, and dried; then dissolved ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistance | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com