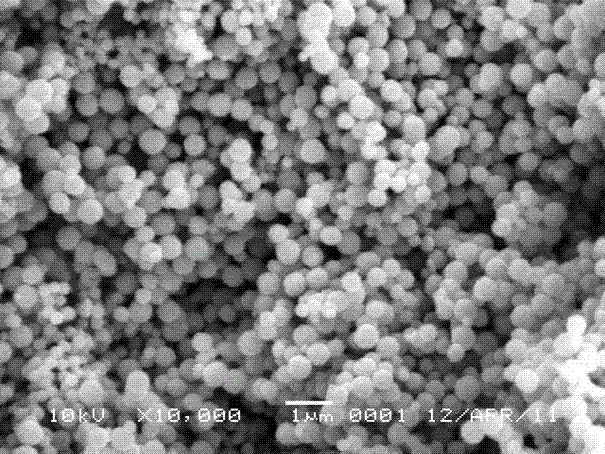
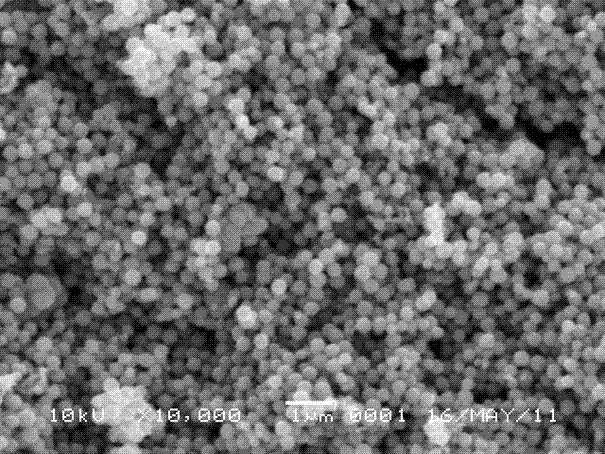
Preparation method of spherical ferroferric oxide
A technology of spherical ferric oxide, which is applied in the field of preparation of spherical ferric ferric oxide by solvothermal method, can solve problems such as insufficient synthesis methods, and achieve the effect of uniform shape, simple process and controllable size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] According to the preparation process, under normal temperature and pressure, 3×10 -3 mol FeCl 3 ·6H 2 O was added to 25 mL of ethylene glycol, and ferric chloride hexahydrate was completely dissolved by magnetic stirring to obtain a clear solution. will be 1.5×10 -3 mol of ammonium acetate was added to the above clear solution, and under the action of magnetic stirring, it was thoroughly mixed to obtain a brown clear solution. The brown clear solution was sealed and placed in a polytetrafluoroethylene-lined stainless steel reaction kettle with a volume of 30 mL, and the reaction kettle was placed in an oven, and the temperature was raised to 200 °C at a heating rate of 5 °C / min. The temperature was reacted for 24 h. After the reaction, the obtained mixture was separated by a centrifuge at a rate of 10000 r / min for 1 min, and the obtained black solid was washed with absolute ethanol for more than 5 times, and the obtained solid was dried in an oven at 80 °C. The d...
Embodiment 2
[0024] According to the preparation process, under normal temperature and pressure, 3×10 -3 mol FeCl 3 ·6H 2 O was added to 25 mL of ethylene glycol, and ferric chloride hexahydrate was completely dissolved by magnetic stirring to obtain a clear solution. will be 1.5×10 -3 mol of ammonium acetate was added to the above clear solution, and under the action of magnetic stirring, it was thoroughly mixed to obtain a brown clear solution. The brown clear solution was sealed and placed in a polytetrafluoroethylene-lined stainless steel reactor with a volume of 30 mL, and the reactor was placed in an oven, and the temperature was raised to 195 °C at a heating rate of 5 °C / min. The temperature was reacted for 24 h. After the reaction, the obtained mixture was separated by a centrifuge at a rate of 10000 r / min for 1 min, and the obtained black solid was washed with absolute ethanol for more than 5 times, and the obtained solid was dried in an oven at 80 °C. The drying time is 8 ...
Embodiment 3
[0026] According to the preparation process, 1×10 -3 mol FeCl 3 ·6H 2 O was added to 25 mL of ethylene glycol, and ferric chloride hexahydrate was completely dissolved by magnetic stirring to obtain a clear solution. will be 1.5×10 -3 mol of ammonium acetate was added to the above clear solution, and under the action of magnetic stirring, it was thoroughly mixed to obtain a brown clear solution. The brown clear solution was sealed and placed in a polytetrafluoroethylene-lined stainless steel reaction kettle with a volume of 30 mL, and the reaction kettle was placed in an oven, and the temperature was raised to 190 °C at a heating rate of 5 °C / min. The temperature was reacted for 12 h. After the reaction was completed, the obtained mixture was separated by a centrifuge at a rate of 10000 r / min for 1 min, and the obtained black solid was washed with absolute ethanol for more than 5 times, and the obtained solid was dried in an oven at 80 °C. The drying time is 8 hours, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com