Method for synthesizing benzyl cyanide compound by using benzyl chloride compound
A compound, the technology of phenylacetonitrile, which is applied in the field of synthesis of phenylacetonitrile compounds, can solve the problems of high industrial application cost and high toxicity of cyanide reagents, and achieve the effects of low toxicity, low price and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
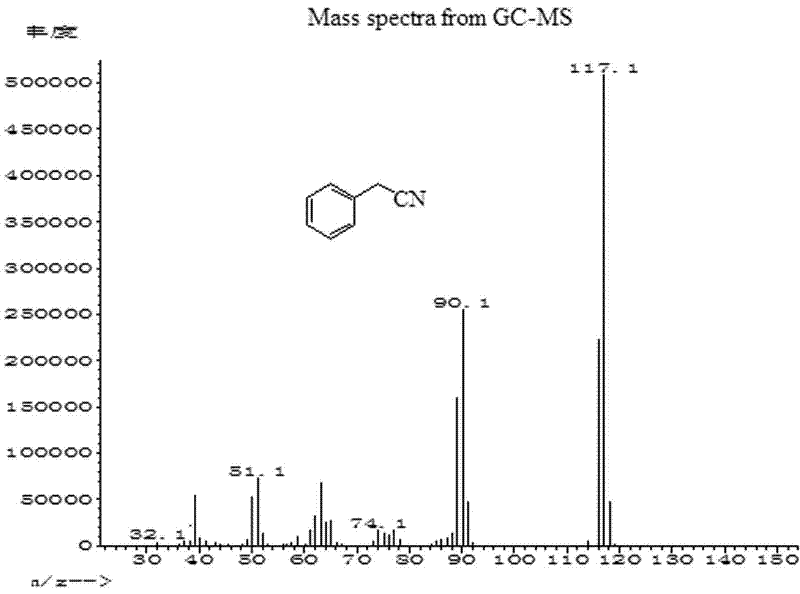
[0027] Add 0.3mmol cuprous iodide and 0.3mL toluene solvent into the reaction tube, stir for 1 minute; then add 0.5mmol K 4 [Fe(CN) 6 ], 1 mmol benzyl chloride and 0.7 mL toluene. After sealing the reaction tube, it was stirred at 180° C. for 20 h. After the reaction system was cooled to room temperature, 1 mL of acetophenone in dichloromethane (0.8 mmol / mL) was added as an internal standard, stirred evenly and left for more than 1 hour, and the supernatant was analyzed by gas chromatography. The yield of phenylacetonitrile was 78%, phenylacetonitrile (C 8 h 7 N) mass spectrum such as figure 1 As shown, the theoretical value of its molecular ion peak (M+) is 117.06, and the measured value is 117.1.
Embodiment 2
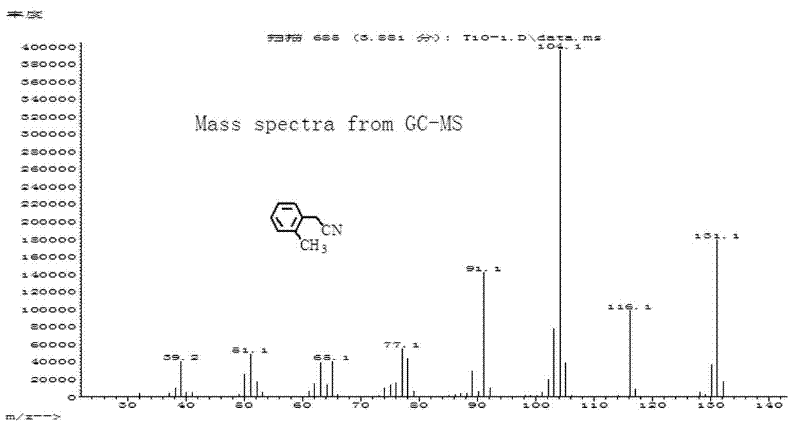
[0029] Add 0.3mmol cuprous iodide and 0.3mL toluene solvent into the reaction tube, stir for 1 minute; then add 0.5mmol K 4 [Fe(CN) 6 ], 1 mmol o-methylbenzyl chloride and 0.7 mL toluene. After sealing the reaction tube, it was stirred at 180° C. for 20 h. After the reaction system was cooled to room temperature, 1 mL of acetophenone in dichloromethane (0.8 mmol / mL) was added as an internal standard, stirred evenly and left for more than 1 hour, and the supernatant was analyzed by gas chromatography. The yield is 82%. O-tolueneacetonitrile (C 9 h 9 N) mass spectrum such as figure 2 As shown, the theoretical value of the molecular ion peak (M+) is 131.07, and the measured value is 131.1.
Embodiment 3
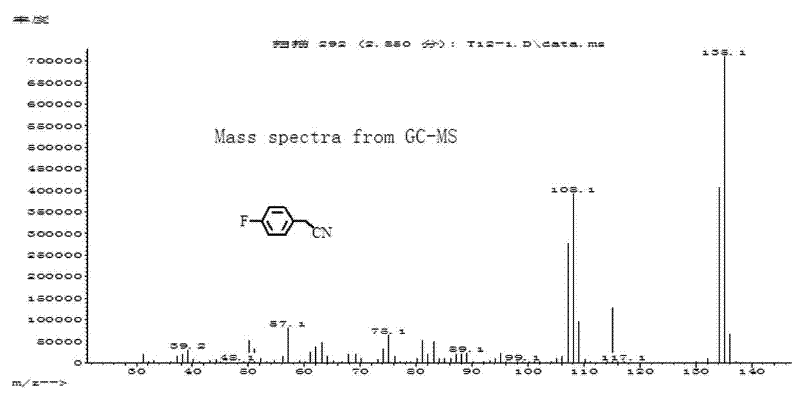
[0031] Add 0.3mmol cuprous iodide and 0.3mL toluene solvent into the reaction tube, stir for 1 minute; then add 0.5mmol K 4 [Fe(CN) 6 ], 1 mmol m-methylbenzyl chloride and 0.7 mL toluene. After sealing the reaction tube, it was stirred at 180° C. for 20 h. After the reaction system was cooled to room temperature, 1 mL of acetophenone in dichloromethane (0.8 mmol / mL) was added as an internal standard, stirred evenly and left for more than 1 hour, and the supernatant was analyzed by gas chromatography. The yield is 76%. m-tolueneacetonitrile (C 9 h 9 N) Mass spectrum, molecular ion peak (M+) theoretical value 131.07, measured value 131.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com