Production method of C5 enol
A production method, the technology of carbopentaenol, which is applied in the field of carbapentaenol production, can solve the problems of high production cost, achieve the effects of reducing production cost, simplifying the process, and increasing the overall reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~8
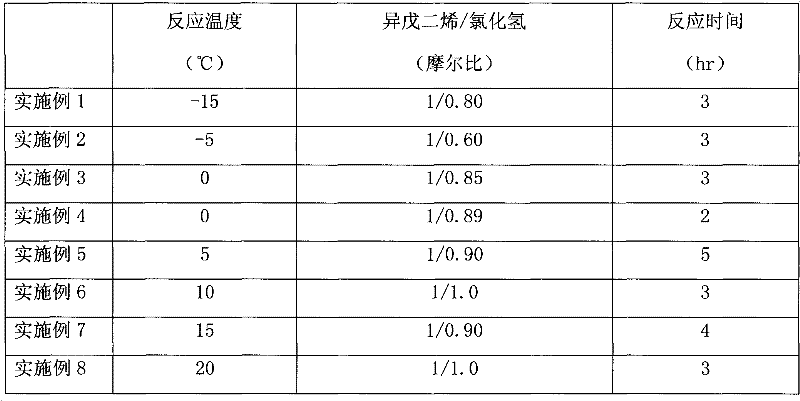
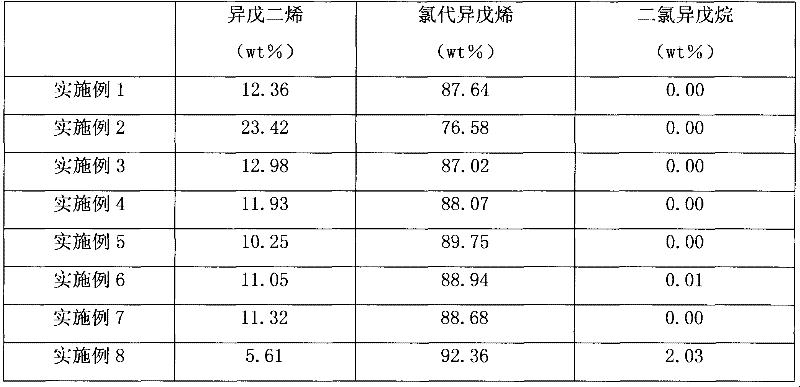
[0026] The addition reaction was carried out in a 1000 ml glass three-neck flask with mechanical stirring and cooling device. Add isoprene into the three-necked flask, start stirring and cool the reaction liquid to the required temperature, then pass the raw material anhydrous hydrogen chloride gas into the reactor from the lower part at this temperature, keep the required reaction time, and make the hydrogen chloride and Isoprene undergoes an addition reaction. Since the addition reaction is an exothermic process, the gas inlet rate of hydrogen chloride should be controlled to maintain the reaction temperature. The reaction conditions of the above embodiments are shown in Table 1, and the reaction results are shown in Table 2.
Embodiment 9~16
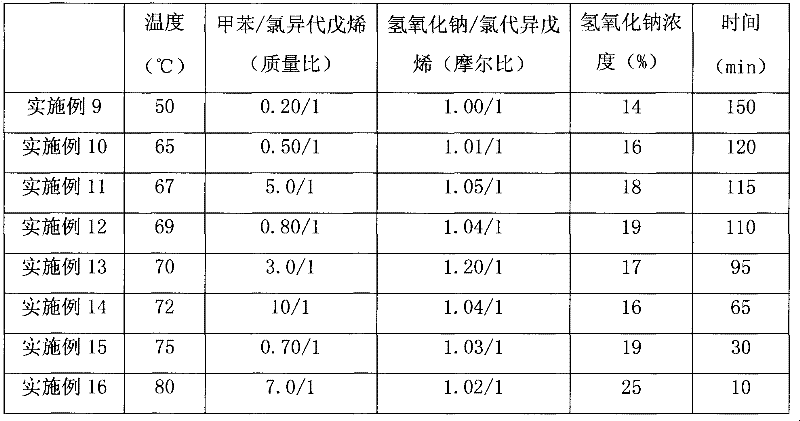
[0028] The hydrolysis reaction was carried out in a 1000 ml glass three-neck flask equipped with a mechanical stirring and heating device. Add chloroisoamylene, toluene and sodium hydroxide aqueous solution into the three-necked flask, start stirring and heat the reaction liquid to the required temperature, and keep the required reaction time. The reaction conditions of the above embodiments are shown in Table 3, and the reaction results are shown in Table 4.
[0029] When refining methyl butenol, the temperature at the top of the tower is 70-72°C, the temperature at the bottom of the tower is controlled at 80-90°C, the absolute pressure at the top of the tower is controlled at 260-270mmHg, and the reflux ratio is controlled at 1-3. When prenol is refined, the temperature at the top of the tower is 75-77°C, the temperature at the bottom of the tower is 85-95°C, the absolute pressure at the top of the tower is controlled at 60-75mmHg, and the reflux ratio is controlled at 1-3. ...
Embodiment 17~23
[0031]The hydrolysis reaction was carried out in a 1000 ml glass three-neck flask with mechanical stirring and heating device. Add chloroisoamylene, solvent and sodium hydroxide aqueous solution into a three-necked flask, start stirring and heat the reaction solution to the required temperature, and keep the required reaction time. The reaction conditions of above each embodiment see the table below:
[0032] Table 1.
[0033]
[0034] Table 2.
[0035]
[0036] table 3.
[0037]
[0038] Table 4.
[0039]
[0040] table 5:
[0041]
[0042] Table 6:
[0043]
PUM
| Property | Measurement | Unit |
|---|---|---|
| oxygen consumption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com