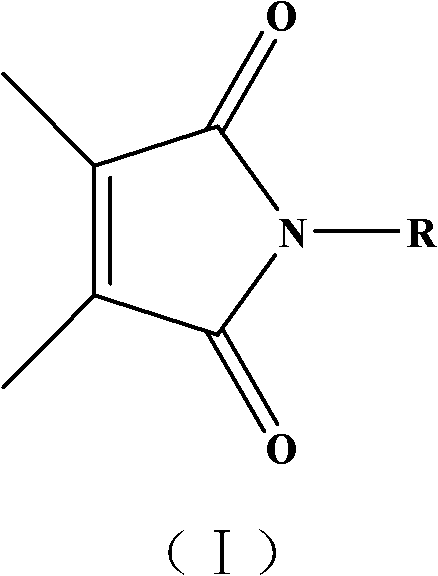
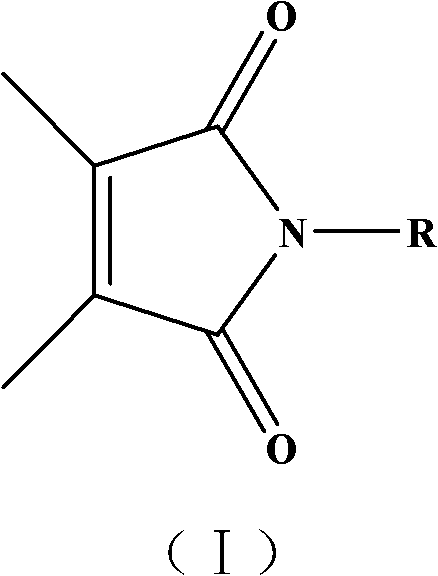
Maleimide compound, its preparation and application
A technology of maleimide and dimethyl maleic anhydride is applied to maleimide compounds and their preparation, and the application field in preventing and treating rape sclerotinia, and can solve the problem of affecting enzymes , affecting the growth of bacteria and other problems, to achieve the effect of simple preparation method, convenient operation and improved inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
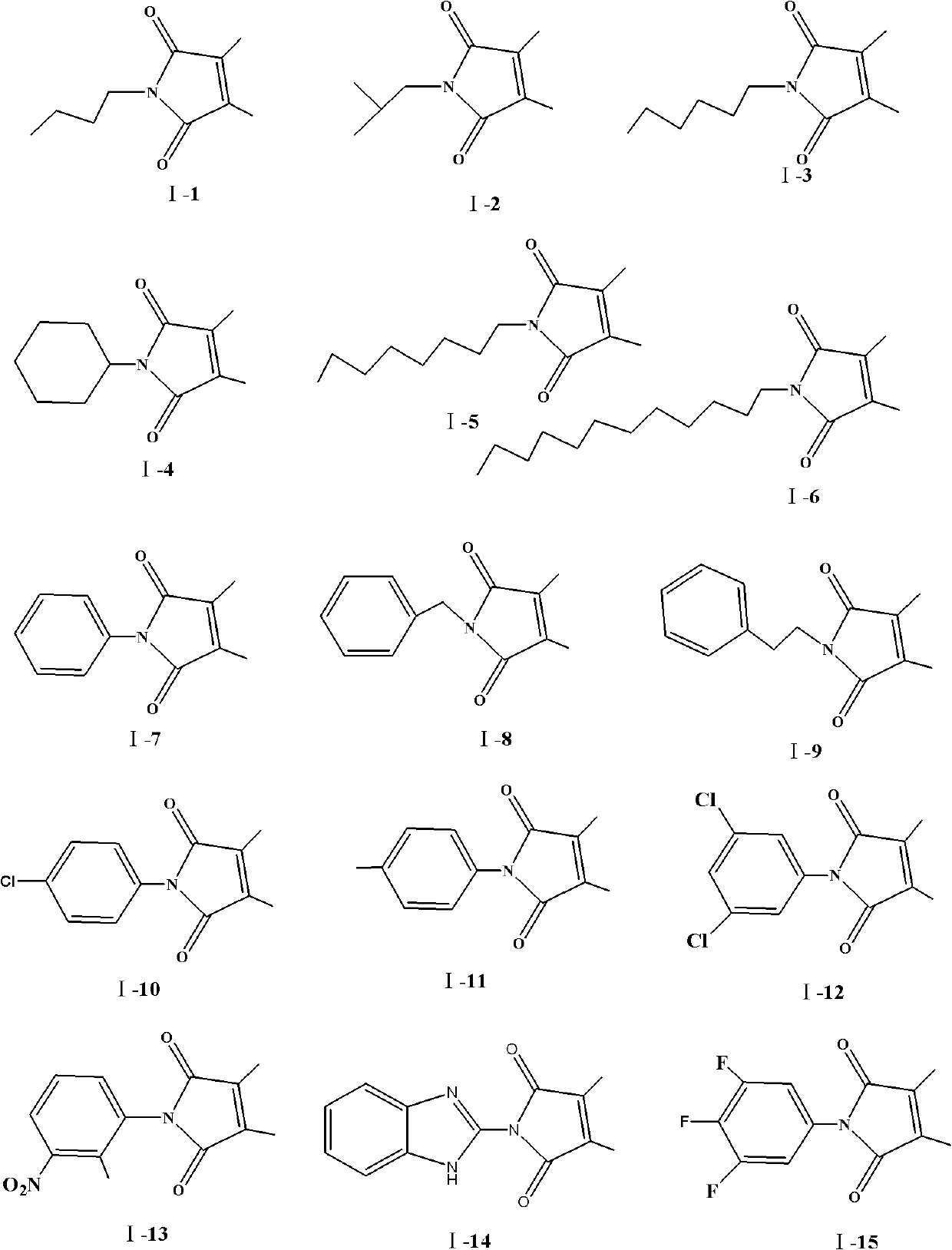
[0022] Embodiment 1: N-n-butyl-2,3-dimethylmaleimide (I-1)
[0023] Weigh 5.0g (0.04mol) of 2,3-dimethylmaleic anhydride (dimethylmaleic anhydride) into a three-necked flask and dissolve it with 30mL of acetone, measure 3.7mL (0.038mol) of n-butylamine, and use Dilute with 20mL of acetone, then add dropwise into the three-neck flask with a constant pressure dropping funnel, and stir while adding dropwise, for about 8 minutes. The system was heated to 35°C and stirred for 3.0h, cooled to 0°C, and then added 2.28g of sodium acetate in triethylamine (0.1g NaAc and 3.0mL of triethylamine), 0.1g of CuI, and 8.0mL of acetic anhydride, stirred evenly, and heated To 80 ° C, stirring and reflux reaction for 6h. After the reaction, the reaction solution was cooled to room temperature (25° C.), part of the acetone in the reaction solution was evaporated, extracted three times with 50 mL of n-hexane, the organic phases were combined and distilled under reduced pressure to obtain a yellow...
Embodiment 2
[0027] Embodiment 2: N-isobutyl-2,3-dimethylmaleimide (I-2)
[0028]Weigh 5.0g (0.04mol) of dimethyl maleic anhydride and add it to a three-necked flask and dissolve it with 30mL of acetone, measure 3.8mL (0.038mol) of isobutylamine, dilute it with 20mL of acetone, and add it dropwise to In the three-neck flask, stir while adding dropwise for about 10 minutes. The system was heated to 45°C and stirred for 4.0h, cooled to 0°C, and 2.76g of sodium acetate in triethylamine solution (0.15g NaAc and 3.6mL of triethylamine), 0.1g of CuI, and 8.5mL of acetic anhydride were added in sequence, stirred evenly, and heated To 70 ° C, stirred and refluxed for 6.5 hours. After the reaction, the reaction solution was cooled to room temperature, part of the acetone in the reaction solution was evaporated, extracted three times with 50 mL of n-hexane, the organic phases were combined and distilled under reduced pressure to obtain a reddish-brown oily concentrated solution. The concentrated s...
Embodiment 3
[0032] Embodiment 3: N-n-hexyl-2,3-dimethylmaleimide (I-3)
[0033] Weigh 2.5g (0.02mol) of dimethyl maleic anhydride into a three-necked flask and dissolve it with about 20mL of toluene, measure 2.5mL (0.019mol) of n-hexylamine, dilute it with about 10mL of toluene, and add it dropwise to In the three-necked flask, stir while adding dropwise, for about 15 minutes, stir and react at 40° C. for 4.0 hours, and obtain a bright yellow liquid. Add 0.81 g of sodium acetate in triethylamine solution (0.08 g NaAc and 1.0 mL triethylamine), 0.08 g hydroquinone, and 3.0 mL acetic anhydride in sequence, stir evenly, heat to 75° C., and reflux at constant temperature for 8.0 h. After the reaction, the reaction solution was washed with water several times until the water layer was neutral, and the toluene layer was washed with anhydrous Na 2 SO 4 Dry, then use activated carbon and silica gel to absorb and decolorize by heating, filter, and concentrate the filtrate under reduced pressure ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com