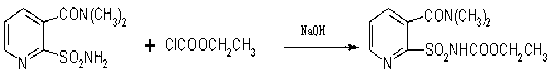Method for synthesizing 2-ethoxycarbonylaminosulfonyl-N,N-dimethyl nicotinamide
A technology of ethoxycarbonylsulfamoyl and dimethylnicotinamide, which is applied in the field of pesticides, can solve the problems of difficult solvent recovery, large environmental pollution, and environmental pollution of potassium carbonate, and achieve simple and thorough solvent separation and economic benefits Significant, cost-saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In a 500ml four-neck flask equipped with a thermometer, a stirrer, a dropping funnel, and a condenser tube, add 0.1mol of 2-aminosulfonamide-N,N-dimethylnicotinamide, 60mL of acetone and 40ml of water, and put the Adjust the temperature to about 10°C, and at the same time add 0.16mol ethyl chloroformate and liquid caustic soda dropwise to the reactant, measure the pH value every 10 minutes during the dripping of liquid caustic soda to ensure that the pH value is between 7.5-8.5, such as pH If it is too high or too low, you can stop the dripping of liquid caustic soda or reduce the rate of addition of ethyl chloroformate. Use a condenser tube to control the temperature at 10-15°C to ensure that the addition of ethyl chloroformate is complete, then continue to keep warm for 2 hours, and take a sample to measure the residue. When the content of 2-aminosulfonamide-N,N-dimethylnicotinamide is less than 0.1%, it means the end of the reaction. Raise the temperature, carry out...
Embodiment 2
[0030] In a 500ml four-neck flask equipped with a thermometer, a stirrer, a dropping funnel and a condenser tube, add 0.1mol of 2-aminosulfonamide-N,N-dimethylnicotinamide, 70mL of the acetone solution recovered in Example 1, 30ml of water, lower the temperature to 10°C, add liquid caustic soda and 0.15mol of ethyl chloroformate to the reactant at the same time, after dripping at 10-15°C, continue to keep warm for 2 hours, take samples and measure the residual 2-aminosulfonamide-N, The content of N-dimethylnicotinamide is less than 0.1%. After the reaction is completed, the temperature is raised and the atmospheric pressure is distilled. After recovering the acetone, water is added to adjust the pH value to acidity, and then filtered, washed with water, and dried to obtain the product. The waste water can be discharged after the factory's biochemical treatment reaches the standard, which is basically non-polluting to the environment. The yield of the product calculated by 2-a...
Embodiment 3
[0032] In a 500ml four-necked flask equipped with a thermometer, a stirrer, a dropping funnel and a condenser, add 0.13mol of 2-aminosulfonamide-N,N-dimethylnicotinamide, 70mL of the acetone solution recovered in Example 2, Add 40ml of water, add 20 mL of fresh acetone, lower the temperature to 10°C, add liquid caustic soda and 0.195mol of ethyl chloroformate to the reactant at the same time, after dropping at 10-15°C, continue to keep warm for 2 hours, take samples and measure the residual The content of 2-aminosulfonamide-N,N-dimethylnicotinamide is less than 0.1%. After the reaction is completed, the temperature is raised and the atmospheric pressure is distilled. After recovering the acetone, water is added to adjust the pH value to acidity, and then filtered, washed with water, and dried to obtain the product. The waste water can be discharged after the factory's biochemical treatment reaches the standard, which is basically non-polluting to the environment. The yield of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com