Anti-herpes simplex virus (HSV) active polypeptide of Tibetan Pi scorpion and application thereof
A technology of herpes simplex virus and Tibetan scorpion, applied in the field of oral herpes simplex, genital herpes, Tibetan scorpion anti-herpes simplex virus active polypeptide, herpes zoster topical gel, in the field of treatment and treatment of herpes simplex, and can solve problems such as poor curative effect , to achieve the effect of good curative effect, obvious effect and obvious therapeutic effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Preparation of a Tibetan lute scorpion anti-herpes simplex virus polypeptide gene.
[0033] Proceed as follows:
[0034] A: Extraction of total RNA from Tibetan lute scorpion venom glands (Trizol LS one-step method: Trizol LS was purchased from Invitrogen, USA)
[0035] ①Take 500mg of scorpion gland and grind it into fine powder in liquid nitrogen, add 10ml TRIZOL reagent and mix well, and leave it at room temperature (20-25℃, the same below) for 5 minutes; Minutes, centrifuge at 12,000g for 15 minutes at 4°C; ③Add 1 volume of isopropanol to the water phase, place at room temperature for 10 minutes, and centrifuge at 12,000g for 10 minutes at 4°C to obtain RNA precipitation; ④Wash the precipitate with 5m175% ethanol, and centrifuge at 7,500g for 5 minutes; ⑤ After the RNA precipitate is dried, dissolve it in DEPC-treated water, and incubate at 55-60°C for 10 minutes to completely dissolve the RNA. The whole process was carried out according to the method re...
Embodiment 2
[0058] Example 2: Structural analysis of AHSVP1 polypeptide and its homologous amphipathic polypeptide
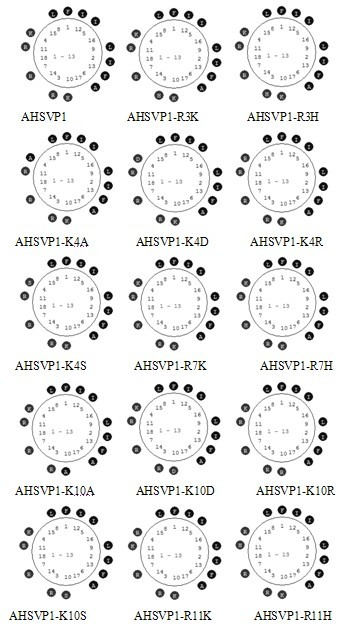
[0059] According to the mature peptide sequence (FIRKIARLLKRIF) of AHSVP1, the online NPSserver [DSC method (Discrimination of protein Secondary structure Class)] was used to predict its secondary structure, and the software AHTHEPROT 2000 was used to display its secondary structure image. The results showed that AHSVP1 contained 100% α-Helix structure, which had a typical amphiphilic α-Helix structure and contained a large number of basic residues (Arg and Lys) with net positive charges. According to the spiral image figure 2 , and then carried out a large number of point mutations to the AHSVP1 polypeptide sequence FIRKIARLLKRIF (Table 1), and found that FIX1X2IAX3LLX4X5IF (X1, X3 and X5 are arbitrary basic amino acids; X2 and X4 are arbitrary amino acids) (SEQ ID NO: 3) sequence does not affect its parent sexual characteristics ( figure 2 ).
Embodiment 3
[0060] Example 3: Chemical synthesis of AHSVP1 polypeptide and its homologous amphipathic polypeptide
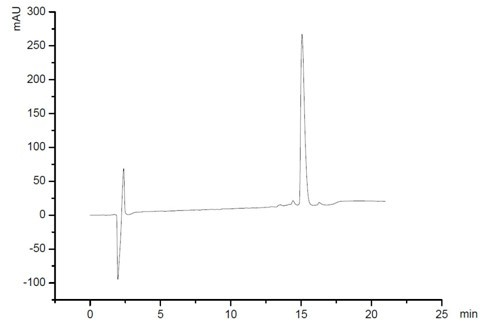
[0061] Artificially synthesized according to the amino acid sequences of AHSVP1 and its homologous amphipathic polypeptides (FIRKIARLLKRIF and FIX1X2IAX3LLSX4IF). The method of solid-phase chemical synthesis has obtained high-purity AHSVP1 polypeptide ( image 3 and Figure 4 ) and its homologous structure amphipathic polypeptide (Table 1).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com