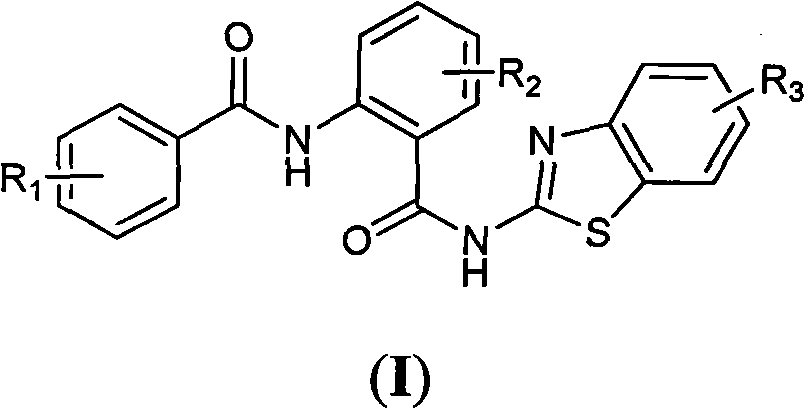
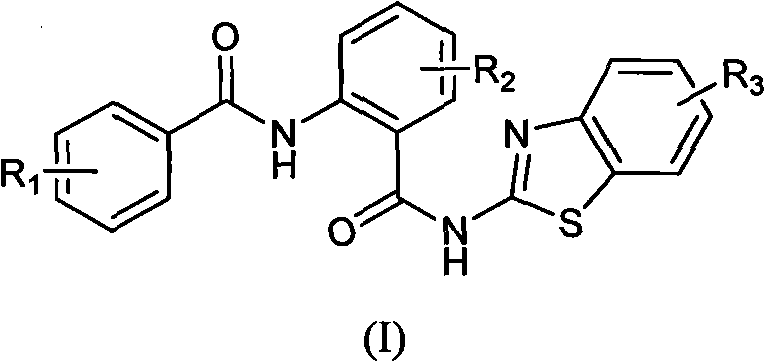
N-(2-(substituted benzo- thiazole-2- amino formacyl) -phenyl group) - benzamide, as well as preparation method and usages thereof
A carbamoyl, benzothiazole technology, applied in N-(2-(substituted benzothiazole-2-carbamoyl)-phenyl)-benzamide and the fields of preparation and use thereof, can solve the inhibitory activity Weak, unstudied, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
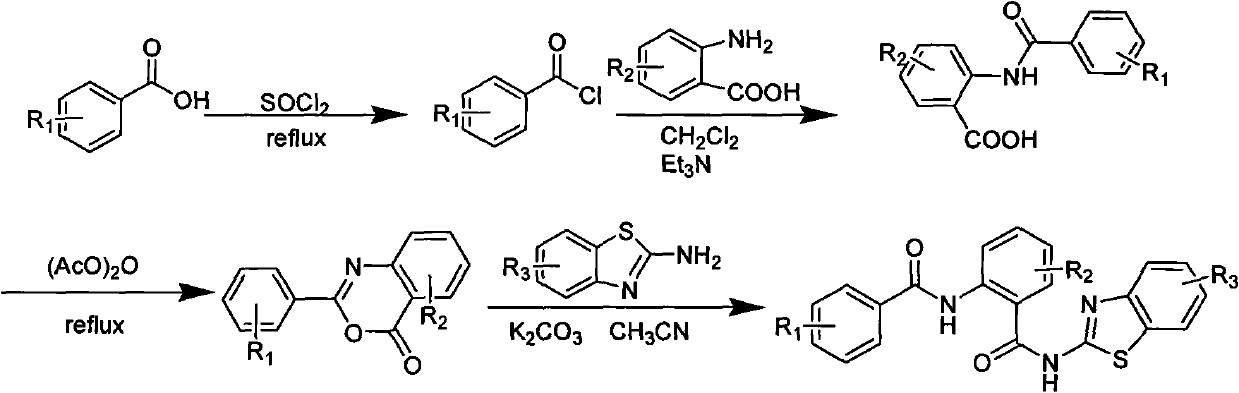
[0035] Example 1. Synthesis of compound N-(2-(6-ethoxybenzothiazole-2-carbamoyl)-phenyl)-2,4-dichlorobenzamide (compound number a):
[0036] (1) Synthesis of 2,4-dichlorobenzoyl chloride
[0037] Get 14.32g (0.075mol) of 2,4-dichlorobenzoic acid in a 100ml there-necked flask (with thermometer, drying tube and tail gas absorption device), then add 20ml of thionyl chloride therein, heat and reflux for 10 hours, TLC After tracking, the reaction was complete, and the excess thionyl chloride was distilled off to obtain 10.99 g of liquid;
[0038] (2) Synthesis of 2-(2,4-dichlorobenzamido)benzoic acid
[0039] Get the anthranilic acid of 8.83g (0.064mol) in the there-necked flask of 250ml, then add the dichloromethane of 30ml in the reaction flask, then add 3ml triethylamine solution thereinto, until the solution is brown and clear, then the After the prepared acid chloride was diluted with 30ml of dichloromethane, it was dropped into the reaction solution drop by drop, and stirre...
Embodiment 2
[0044] Embodiment 2, the synthesis of compound N-(2-(6-methylbenzothiazole-2-carbamoyl)-phenyl)-2,4-dichlorobenzamide (compound number is b)
[0045] (1) Synthesis of 2,4-dichlorobenzoyl chloride
[0046] Synthesize as embodiment one (1) condition and method;
[0047] (2) Synthesis of 2-(2,4-dichlorobenzamido)benzoic acid
[0048] Synthesize as embodiment one (2) condition and method;
[0049] (3) Synthesis of 2-(2,4-dichlorophenyl)-4H-benzo[d][1,3]oxazin-4-one
[0050] Synthesize as embodiment one (3) condition and method;
[0051] (4) Synthesis of N-(2-(6-methylbenzothiazole-2-carbamoyl)-phenyl)-2,4-dichlorobenzamide
[0052] Synthesized under the same conditions and methods as in Example 1 (4), except that 0.33 g of 2-amino-6-methylbenzothiazole was added, and the reaction time was 6 hours to obtain a light yellow solid. Yield: 40%, mp: 228-229°C.
Embodiment 3
[0053] Example 3, the synthesis of compound N-(2-(6-chlorobenzothiazole-2-carbamoyl)-phenyl)-2,4-dichlorobenzamide (compound number is c)
[0054] (1) Synthesis of 2,4-dichlorobenzoyl chloride
[0055] Synthesize as embodiment one (1) condition and method;
[0056] (2) Synthesis of 2-(2,4-dichlorobenzamido)benzoic acid
[0057] Synthesize as embodiment one (2) condition and method;
[0058] (3) Synthesis of 2-(2,4-dichlorophenyl)-4H-benzo[d][1,3]oxazin-4-one
[0059] Synthesize as embodiment one (3) condition and method;
[0060] (4) Synthesis of N-(2-(6-chlorobenzothiazole-2-carbamoyl)-phenyl)-2,4-dichlorobenzamide
[0061] Synthesized under the same conditions and methods as in Example 1 (4), except that 0.37 g of 2-amino-6-chlorobenzothiazole was added, and the reaction time was 9 hours to obtain a white solid. Yield: 37%, mp: >250°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com