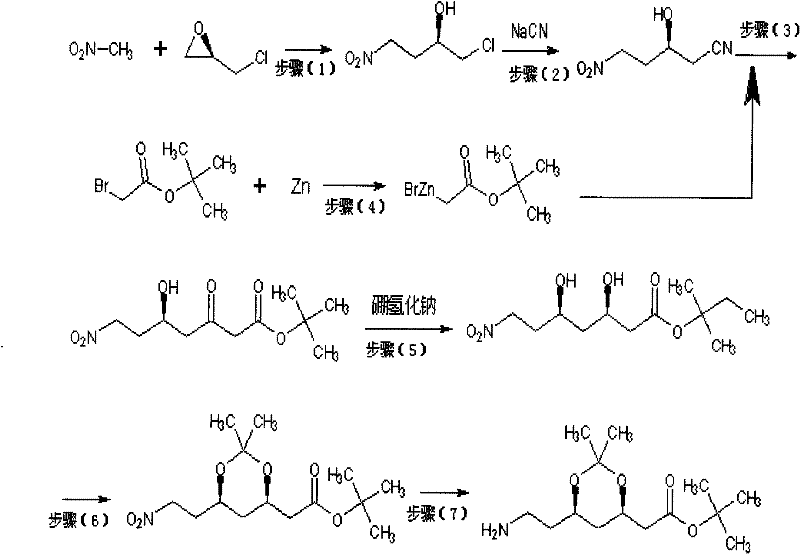
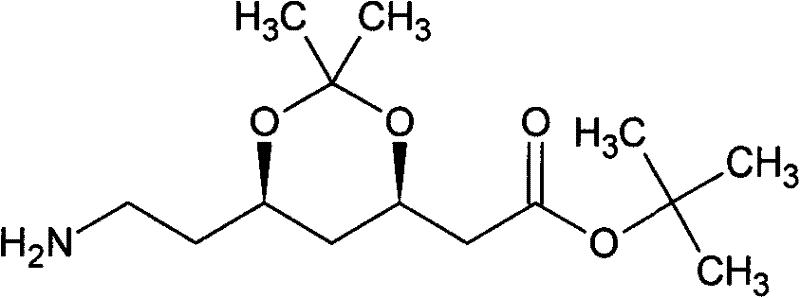
Preparation method of atorvastatin intermediate (3R, 5S)-7-amino-3,5-O-isopropylidene-3,5-dyhydroxyl heptylic acid tert-butyl acetate
A technology of tert-butyl hydroxyheptanoate and atorvastatin, which is applied in the field of preparation of chiral pharmaceutical intermediates, can solve the problems of high industrialization cost, many reaction by-products, long process routes, etc., and achieve easy industrial production and reduce by-products. The effect of simple reaction and synthesis process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
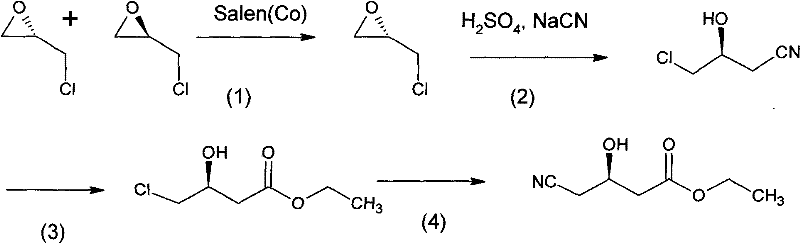
Image
Examples
Embodiment 1
[0032] 1. Add 73.2g of nitromethane and 92.5g of S-propylene oxide into the reaction flask, feed 1.0g of boron trifluoride gas, stir and react at 20°C for 5 hours, detect with gas chromatography, when S-epoxy After the complete conversion of chloropropane, the unreacted nitromethane was removed under reduced pressure to obtain 137.7 g of light yellow oily liquid. 1 It was confirmed by H-NMR and MS to be 1-chloro-4-nitro-(S)-2-butanol with a yield of 90.1% and a content of 98.0%.
[0033]2. Add 153g of 1-chloro-4nitro-(S)-2-butanol prepared in step (1), 200ml of 95% ethanol and 500ml of water in the reaction flask, and start to add 30% sodium cyanide dropwise at 0°C The solution is 196g, the reaction is exothermic, the dropwise addition temperature is controlled at 15±5°C, and the dropwise addition is completed in 8 hours. After the dropwise addition, react at a temperature of 15±5°C, and stop the reaction when the reaction of 1-chloro-4nitro-(S)-2-butanol is detected by gas c...
Embodiment 2
[0040] 1. Add 73.2g of nitromethane and 92.5g of S-epoxypropylene into the reaction flask, pass through 2.5g of hydrogen chloride, stir and react at 0°C for 8 hours, detect with gas chromatography, when the S-epoxychlorohydrin is completely converted Rear unreacted nitromethane was removed under reduced pressure to obtain 134.6 g of light yellow oily liquid, and the product structure was verified by 1 It was confirmed by H-NMR and MS to be 1-chloro-4-nitro-(S)-2-butanol with a yield of 88.0% and a content of 98.4%.
[0041] 2. Add 153g of 1-chloro-4nitro-(S)-2-butanol prepared in step (1), 200ml of 95% ethanol and 500ml of water in the reaction flask, heat up to 50°C, and start to add 30% cyanide 196g of sodium chloride solution, the reaction is exothermic, the dropwise addition temperature is controlled at 50±5°C, and the dropwise addition is completed in 2 hours. After the dropwise addition, control the temperature at 50±5°C, and stop the reaction when the reaction of 1-chl...
Embodiment 3
[0048] 1. Add 73.2g nitromethane and 92.5g S-epoxypropylene into the reaction flask, feed 2.5g hydrogen chloride, stir and react at 50°C for 8 hours, detect with gas chromatography, when the S-epoxychlorohydrin is completely converted Rear unreacted nitromethane was removed under reduced pressure to obtain 141.0 g of light yellow oily liquid, and the product structure was verified by 1 It was confirmed by H-NMR and MS to be 1-chloro-4-nitro-(S)-2-butanol with a yield of 92.3% and a content of 98.7%.
[0049] 2. Add 153g of 1-chloro-4nitro-(S)-2-butanol prepared in step (1), 200ml of 95% ethanol and 500ml of water in the reaction flask, heat up to 50°C, and start to add 30% cyanide 196g of sodium chloride solution, the reaction is exothermic, the dropwise addition temperature is controlled at 55±5°C, and the dropwise addition is completed in 2 hours. After the dropwise addition, the temperature was raised to 80°C for reaction, and the reaction was stopped when the 1-chloro-4ni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com