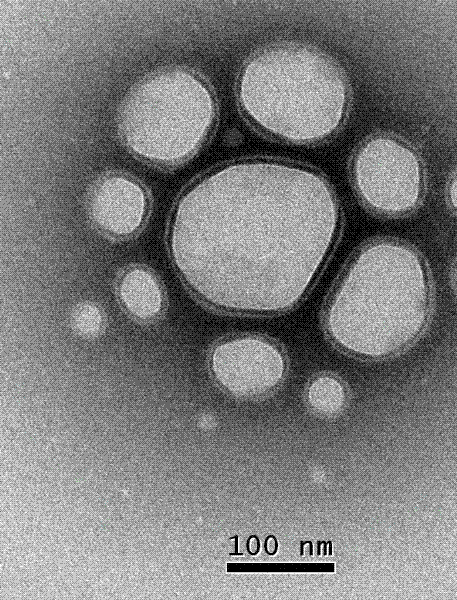
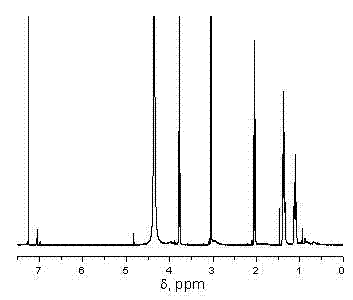
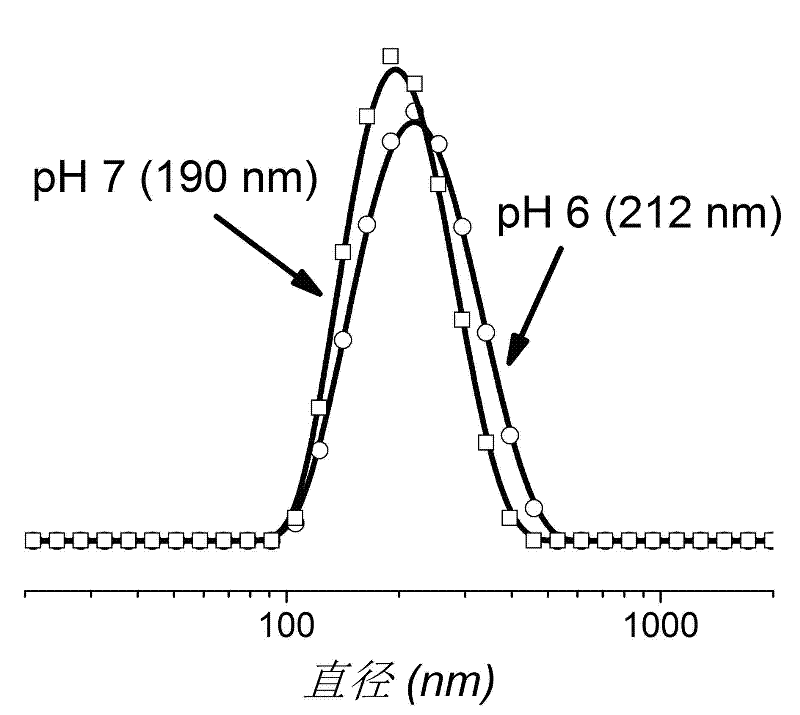
Method for preparing biocompatible polymer nano-vesicle in pure water
A biocompatible, nanovesicle technology, used in pharmaceutical formulations, medical preparations with inactive ingredients, etc., can solve problems such as unfavorable large-scale production and time-consuming dialysis methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1) Amphiphilic block copolymer PCL- b -The specific synthetic scheme of PMPC is as follows:
[0033] a) Ring-opening polymerization to generate macroinitiator PCL-Br
[0034] Add 60 mL of dried anhydrous toluene, 76.3 g of ε-caprolactone, 1.2 g of benzyl alcohol, and 18.8 uL of stannous octoate into a 500 mL round-bottomed flask, 110 O Stir the reaction in an anaerobic state in a C oil bath for 48 hours, then place it at room temperature to cool, continue to add 400 mL of dried toluene, 7.8 mL of dry triethylamine, and 6.8 mL of 2-bromoisobutyryl bromide into the round bottom flask, Stir and react in an ice-water bath for 48 hours, filter, extract, collect the organic phase, dry, filter, precipitate, filter with suction, and dry in vacuum to obtain the macromolecular initiator PCL-Br.
[0035] b) Synthesis of amphiphilic block copolymer PCL- by atom transfer radical polymerization b -PMPC
[0036] Add 1.0 g macroinitiator PCL-Br, 1.6 g monomer MPC, 44.6 mg catalyst ...
Embodiment 2
[0040] 1) Amphiphilic block copolymer PCL- b -The specific synthetic scheme of PMPC is as follows:
[0041] a) Ring-opening polymerization to generate macroinitiator PCL-Br
[0042] Add 60 mL of dried toluene, 76.3 g of ε-caprolactone, 1.2 g of benzyl alcohol, and 18.8 uL of stannous octoate into a 500 mL round-bottomed flask, 110 OStir the reaction in an anaerobic state in an oil bath for 48 hours, then cool at room temperature, and then add 400 mL of dried toluene, 7.8 mL of dry triethylamine, and 6.8 mL of 2-bromoisobutyryl bromide into the round bottom flask , stirred and reacted in an ice-water bath for 48 hours, filtered, extracted, collected the organic phase, dried, filtered, precipitated, suction filtered, and vacuum-dried to obtain the macromolecular initiator PCL-Br.
[0043] b) Synthesis of amphiphilic block copolymer PCL- by atom transfer radical polymerization b -PMPC
[0044] Add 1.0 g macroinitiator PCL-Br, 0.3 g monomer MPC, 44.6 mg catalyst ligand bipyrid...
Embodiment 3
[0048] 1) Amphiphilic block copolymer PCL- b -The specific synthetic scheme of PMPC is as follows:
[0049] a) Ring-opening polymerization to generate macroinitiator PCL-Br
[0050] Add 40 mL of dried toluene, 38.2 g of ε-caprolactone, 0.6 g of benzyl alcohol, and 9.4 uL of stannous octoate into a 250 mL round bottom flask, 110 O Stir the reaction in an anaerobic state in a C oil bath for 48 hours, then cool at room temperature, and then add 400 mL of dried toluene, 3.9 mL of dry triethylamine, and 3.4 mL of 2-bromoisobutyryl bromide into the round bottom flask , stirred and reacted in an ice-water bath for 48 hours, filtered, extracted, collected the organic phase, dried, filtered, precipitated, suction filtered, and vacuum-dried to obtain the macromolecular initiator PCL-Br.
[0051] b) Synthesis of amphiphilic block copolymer PCL- by atom transfer radical polymerization b -PMPC
[0052] Put 1.0 g macroinitiator PCL-Br, 0.8 g monomer MPC, 89.2 mg catalyst ligand bipyridi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com