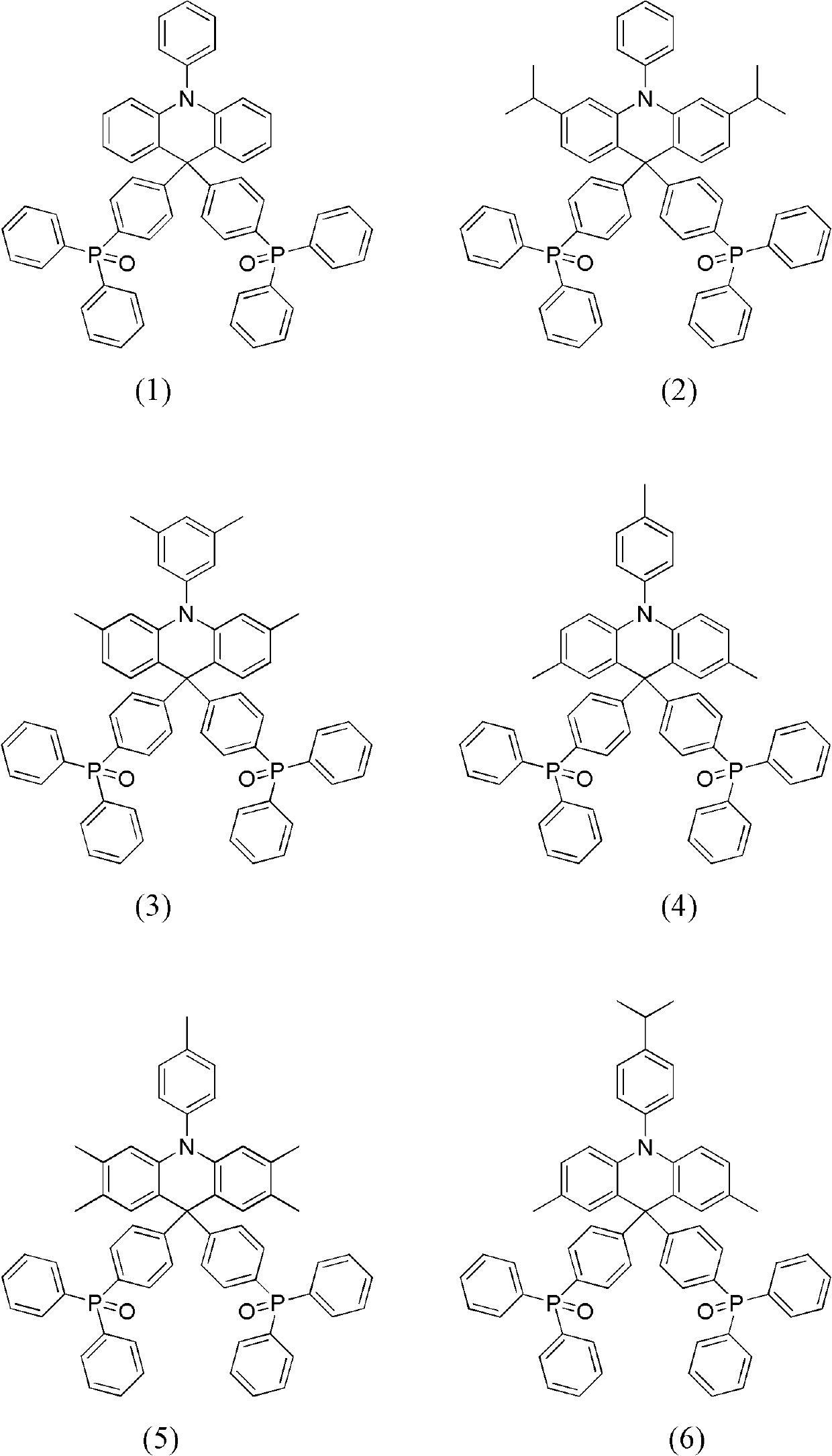
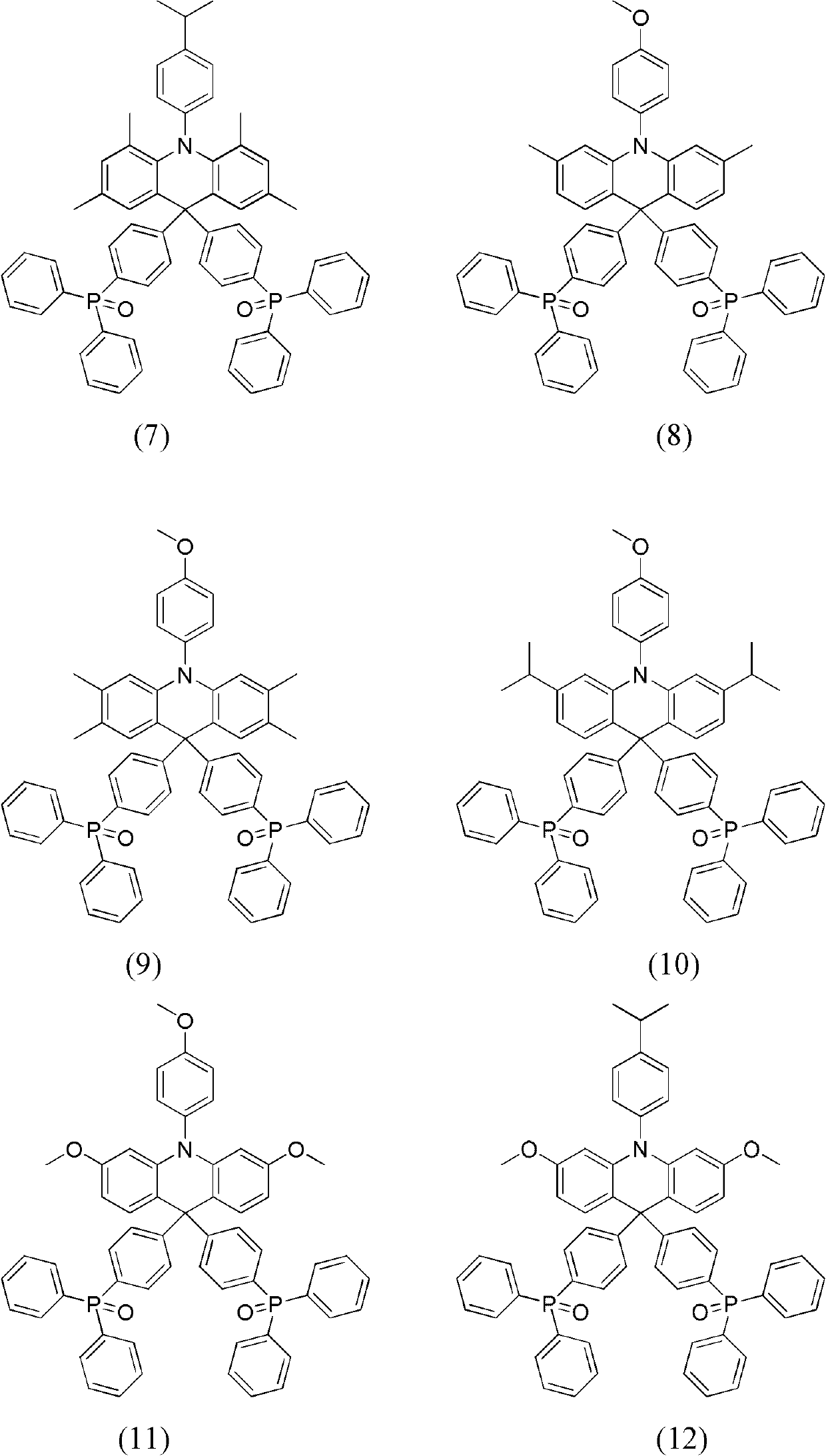
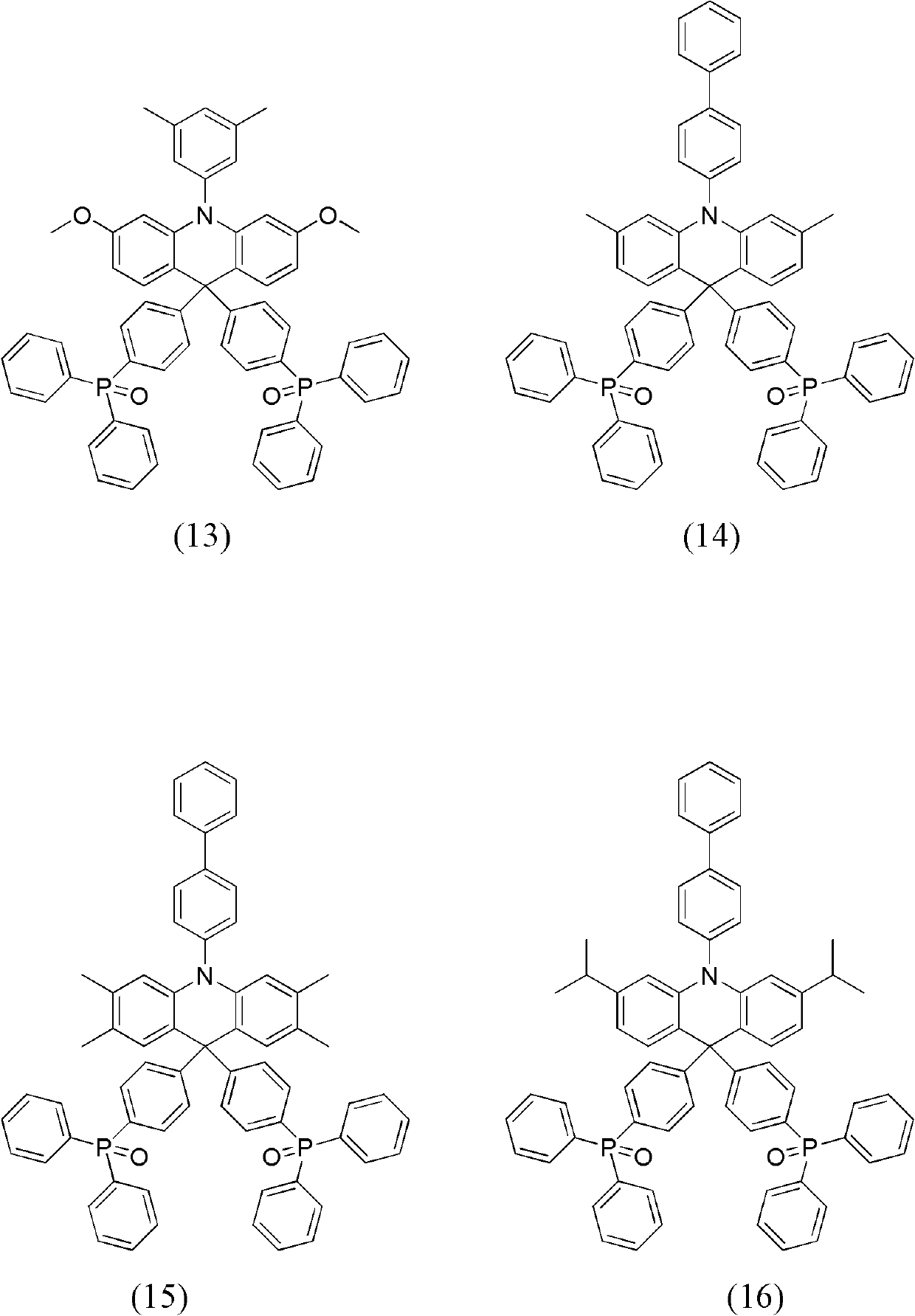
Dihydro acridine derivatives, application thereof and organic electroluminescent device applying same
A technology of dihydroacridine derivatives, which is applied in the field of dihydroacridine derivatives, can solve the problems of luminescence quenching and high cost, and achieve the effects of reducing the turn-on voltage and high current efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The preparation method of the dihydroacridine derivative substituted by diphenylphosphine oxide of the present invention will be described in detail below. The preparation method includes the following steps.
[0038] In the first step, palladium dibenzylideneacetone (Pd(dba) 2 ), tri-tert-butylphosphine (P(t-Bu) 3 ), in the presence of sodium tert-butoxide (NaO(t-Bu)), aniline or diphenylamine or their derivatives, and o-dibromobenzene or 1-bromo-2-iodobenzene or their derivatives, according to the reaction The substitution reaction is carried out at a molar ratio of 1:1.2 or 1:2.2 to obtain triarylamine (intermediate A).
[0039] In the second step, under the system of anhydrous tetrahydrofuran (THF) as the solvent, under the temperature condition of -78°C, add n-butyllithium (n-BuLi) to the intermediate A according to the reaction molar ratio of 1:1.2 or 1 : 2.2 is reacted to obtain the corresponding lithium salt. A THF solution of 4,4'-dibromobenzophenone was ad...
Embodiment 1
[0053] The synthesis of embodiment 1 compound (1)
[0054]
[0055] (1) Synthesis of intermediate (1-A)
[0056] Under nitrogen protection, add 33.8g (0.2mol) diphenylamine, 56.64g (0.24mol) o-dibromobenzene, 0.58g (0.5mol%) Pd (dba) in a 500ml three-neck round bottom flask 2 , 2.0ml (0.5mol%) P(t-Bu) 3 10% cyclohexane solution and 19.2g (0.2mol) NaO(t-Bu), and then 300ml of anhydrous-treated toluene was added to obtain a reaction solution. The above reaction solution was refluxed in an oil bath at 110°C for 4 hours under magnetic stirring, cooled, and then the reaction solution was washed with an appropriate amount of water for 2-3 times before liquid separation, and the obtained organic phase was washed with anhydrous MgSO 4 After drying, the organic solvent was removed by rotary evaporation to obtain the crude product. The crude product was separated by silica gel (200-300 mesh, Qingdao Ocean Chemical Factory) column chromatography to obtain 44.12 g of white crystals,...
Embodiment 2
[0065] The synthesis of embodiment 2 compound (4)
[0066]
[0067] Using 4,4'-dimethyldiphenylamine instead of diphenylamine, and 1-bromo-2-iodo-5-methylbenzene instead of o-dibromobenzene, compound (4) was obtained through the same steps as in Example 1. Yield 88.6%.
[0068] Compound (4) product MS (m / z): 851; elemental analysis (C 58 h 47 NO 2 P 2 ): theoretical value C: 81.77%, H: 5.56%, N: 1.64%; measured value C: 81.70%, H: 5.52%, N: 1.62%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com