Preparation method for amphiphilic konjac glucomannan cholesterol grafted polymer and application
A konjac glucomannan and cholesterol technology is applied in the preparation field of drug carrier material, carboxymethyl konjac glucomannan cholesterol graft, can solve problems such as unfavorable health, and achieve good biocompatibility and reaction conditions Gentle, easy-to-implement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
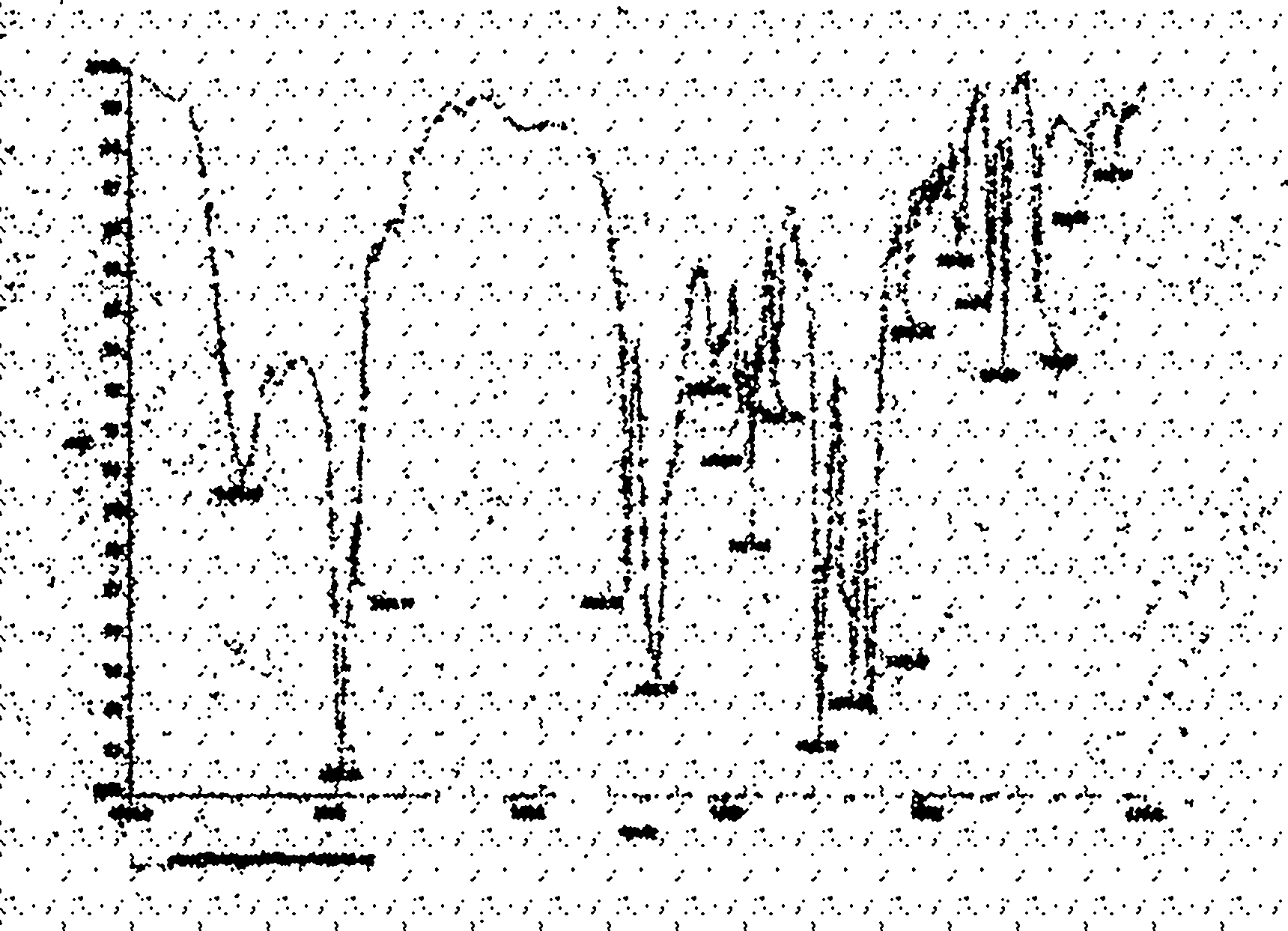
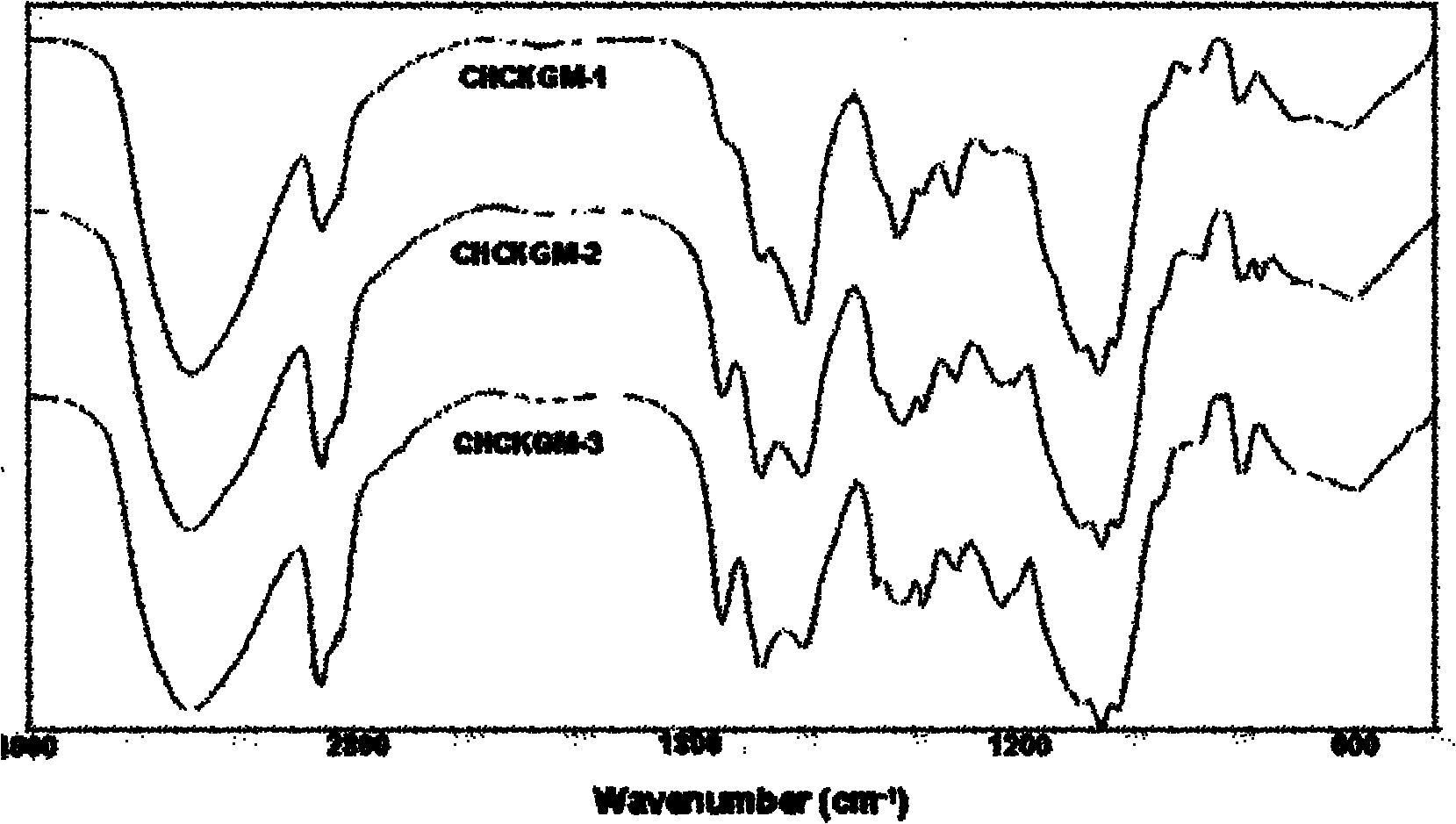
[0027] The preparation of embodiment 1CHCKGM graft
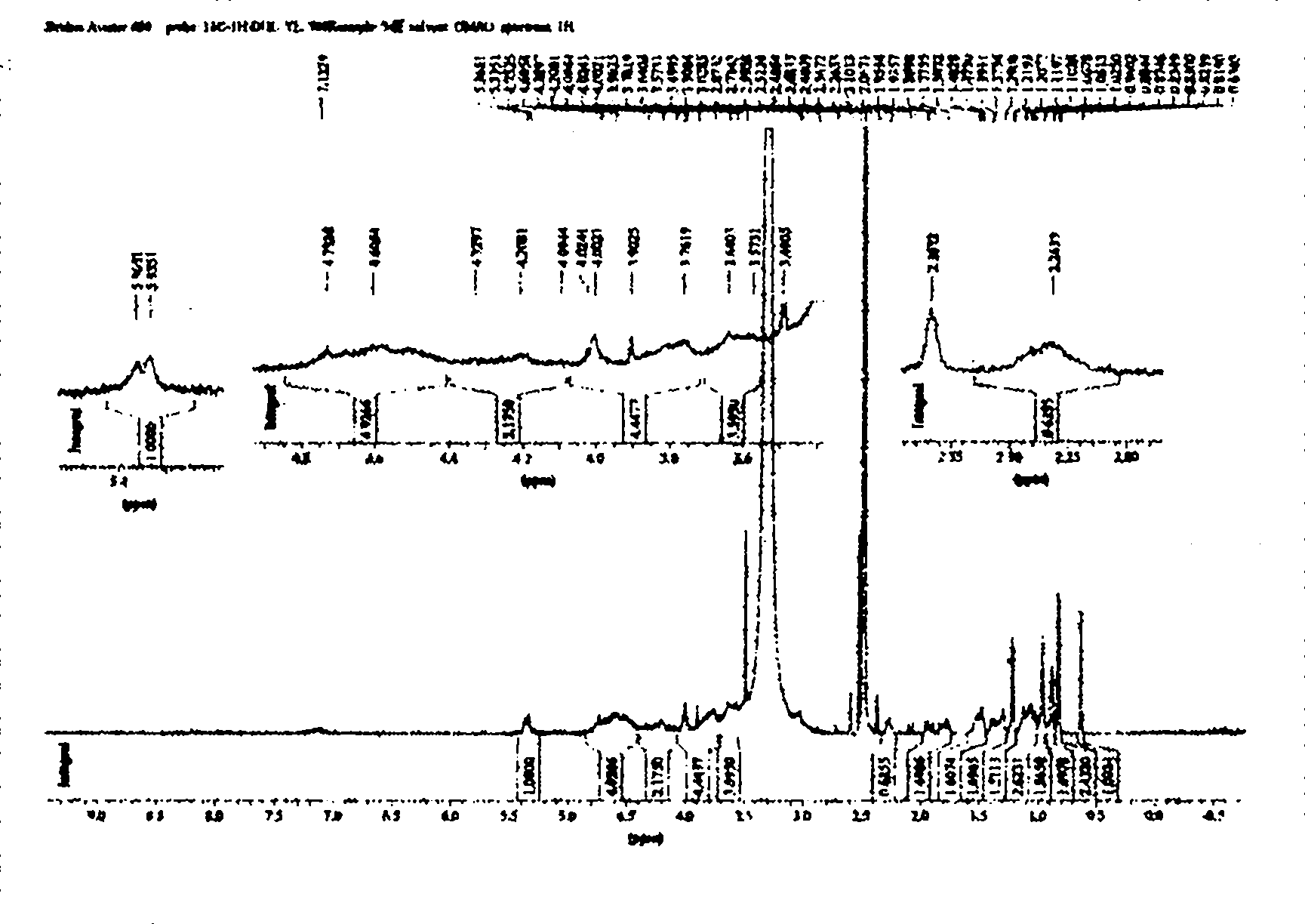
[0028] (1) Synthesis of N-t-butoxycarbonyl-glycine cholesteryl ester (N-t-glycine-CH): cholesterol 1mmol (0.386g), N-tert-butoxycarbonyl-glycine 1.1mmol (0.1925g), 4-dimethylamino Pyridine 0.25mmol (0.031g), dissolved in 5ml of dichloromethane, then added 1.1mmol of dicyclohexylcarbodiimide (0.226g), stirred in an ice bath, reacted for 24h, after the reaction, filtered to remove the white precipitate Dicyclohexylurea, the solvent is removed at room temperature, the product is dissolved in a small amount of acetone, overnight in the refrigerator, the precipitated DCU is removed by filtration, and the solvent is evaporated to dryness to obtain solid N-tert-butoxycarbonyl-glycine cholesterol ester (N-t-glycine- CH).
[0029] (2) Synthesis of glycine cholesteryl ester (Glycine-CH): Dissolve the product in step (1) in 5 milliliters of dichloromethane, add 5 milliliters of trifluoroacetic acid with stirring in an ice bath, and re...
Embodiment 2
[0031] The preparation of embodiment 2CHCKGM graft
[0032] (1) Synthesis of N-t-butoxycarbonyl-glycine cholesteryl ester (N-t-glycine-CH): cholesterol 1mmol (0.386g), N-tert-butoxycarbonyl-glycine 1.5mmol (0.2625g) 4-dimethylaminopyridine 0.25mmol (0.031g), was dissolved in 10 milliliters of dichloromethane, then added 1.1mmol dicyclohexylcarbodiimide (0.226g), stirred in an ice bath, reacted for 24h, after the reaction was over, filtered to remove the white precipitate di Cyclohexylurea, remove the solvent at room temperature, the product is dissolved in a small amount of acetone, and overnight in the refrigerator, the DCU that is separated out is removed by filtration, and the solvent is evaporated to dryness to obtain solid N-tert-butoxycarbonyl-glycine cholesteryl ester (N-t-glycine-CH ).
[0033] (2) Synthesis of glycine cholesteryl ester (Glycine-CH): Dissolve the product in step (1) in 5 milliliters of dichloromethane, add 5 milliliters of trifluoroacetic acid with st...
Embodiment 3
[0035] The preparation of embodiment 3CHCKGM graft
[0036] (1) Synthesis of N-t-butoxycarbonyl-glycine cholesteryl ester (N-t-glycine-CH): cholesterol 1mmol (0.386g), N-tert-butoxycarbonyl-glycine 1.5mmol (0.2625g) 4-dimethylaminopyridine 0.5mmol (0.062g), dissolved in 5-10ml of dichloromethane, then added 1.1mmol of dicyclohexylcarbodiimide (0.226g), stirred in an ice bath, reacted for 18h, after the reaction, filtered to remove the white Precipitate dicyclohexylurea, remove the solvent at room temperature, dissolve the product in a small amount of acetone, overnight in the refrigerator, filter and remove the precipitated DCU, evaporate the solvent to obtain solid N-tert-butoxycarbonyl-glycine cholesteryl ester (N-t-glycine -CH).
[0037] (2) Synthesis of glycine cholesteryl ester (Glycine-CH): the product in step (1) was dissolved in 5 milliliters of dichloromethane, 5 milliliters of trifluoroacetic acid was added under stirring in an ice bath, and reacted for 3 h under st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com