Method for producing thiosulfate and recovering carbon dioxide by utilizing industrial flue gas
A thiosulfate, carbon dioxide technology, applied in the directions of thiosulfate/dithionite/polythionite, chemical instruments and methods, separation methods, etc., can solve the problem of ammonia consumption, high use cost and production cost Advanced problems, to achieve the effect of simple equipment requirements, realization of recycling, and reduction of secondary pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] To produce ammonium thiosulfate and reclaim carbon dioxide as example the present invention is described in detail:
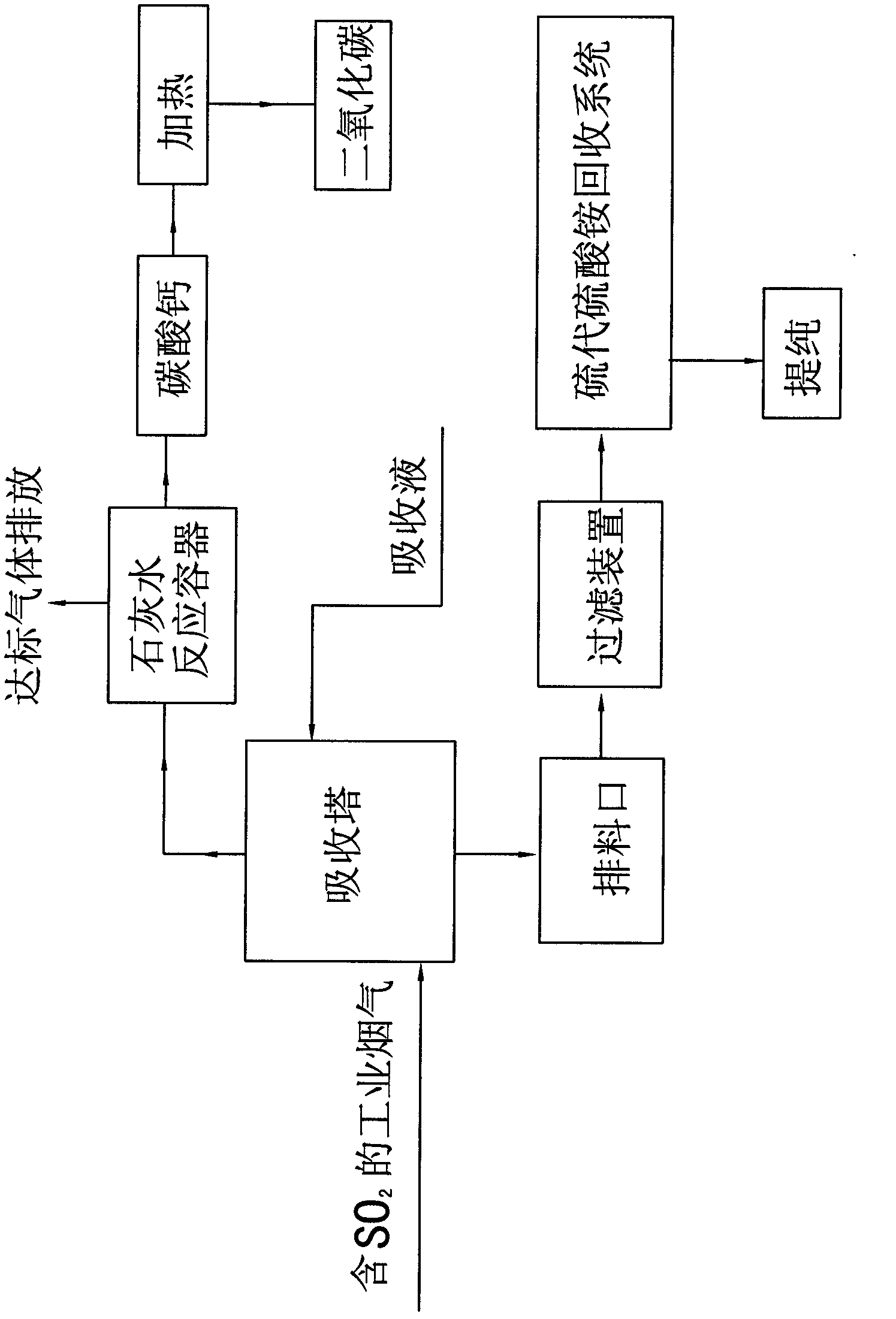
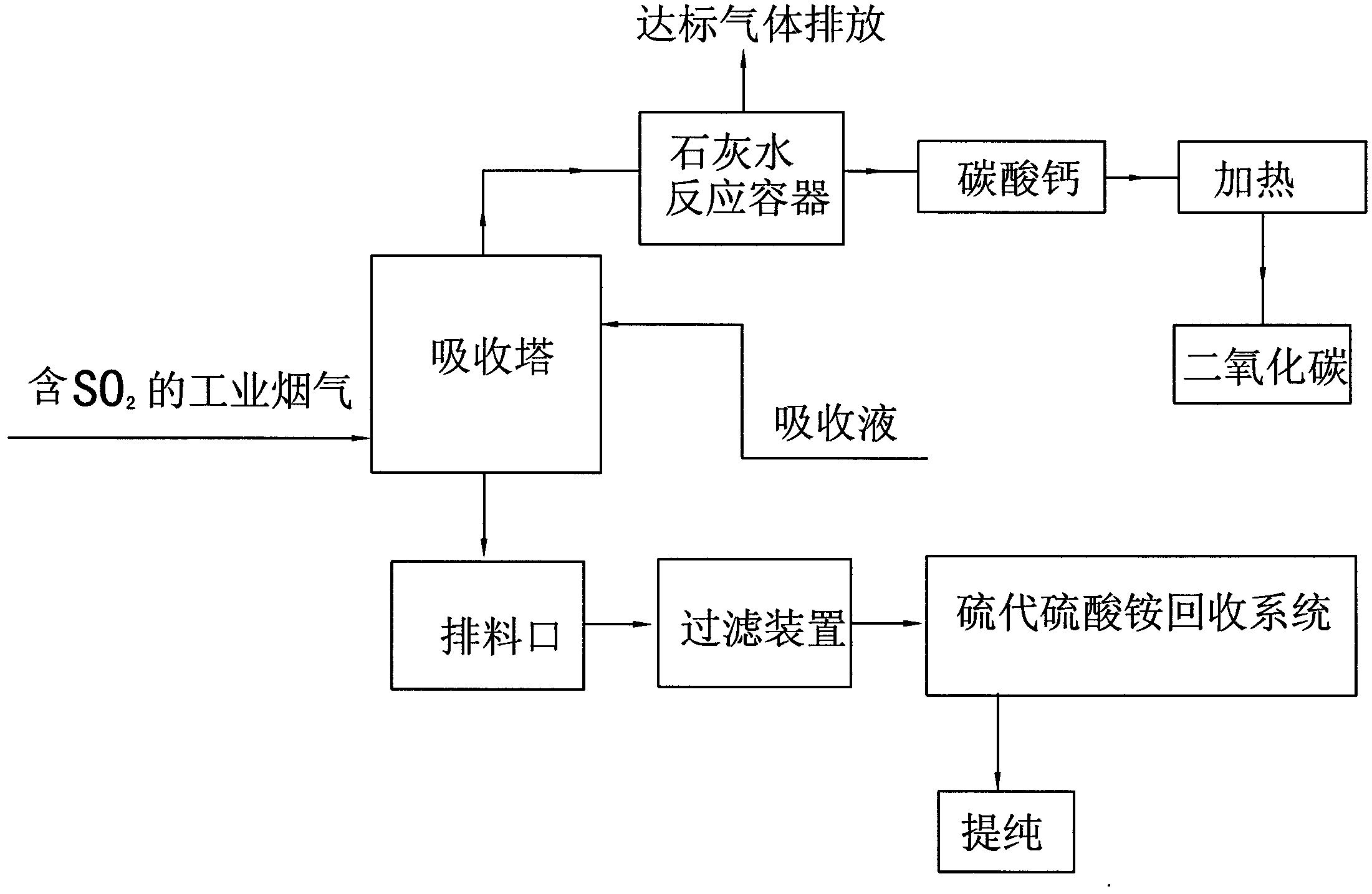
[0025] Example A, such as figure 1 Shown, the present invention utilizes industrial flue gas to produce ammonium thiosulfate and the method for producing pure carbon dioxide, comprises the following steps:
[0026] 1. Add NH 4 HCO 3 Prepare an absorption liquid with a concentration of 16.7% and a pH value of 9.5, fully contact the flue gas containing sulfur dioxide with the absorption liquid in the absorption tower, control the temperature of the flue gas entering the absorption tower at 150°C, and control the concentration of sulfur dioxide at 5000ppm. The sulfur dioxide in the flue gas is dissolved in the absorption liquid, and the absorption reaction formula is:
[0027] 2NH 4 HCO 3 +SO 2 +H 2 O=(NH 4 ) 2 SO 3 +2H 2 O+2CO 2 ;
[0028] Detect the pH value every 3 hours of reaction, when it is detected that the pH value of the solution in t...
Embodiment 2
[0049] Embodiment two, the present invention is described in detail to producing sodium thiosulfate and reclaiming carbon dioxide as an example:
[0050] Such as figure 1 Shown, the present invention utilizes industrial flue gas to produce sodium thiosulfate and the method for producing pure carbon dioxide, comprises the following steps:
[0051] 1. Prepare NaHCO3 into an absorption liquid with a concentration of 15.8% and a pH value of 9.8, and fully contact the flue gas containing sulfur dioxide with the absorption liquid in the absorption tower. The temperature of the flue gas entering the absorption tower is controlled at 120°C and the concentration of sulfur dioxide 3000ppm. The sulfur dioxide in the flue gas is dissolved in the absorption liquid, and the absorption reaction formula is:
[0052] 2NaHCO 3 +SO 2 +H 2 O=Na 2 SO 3 +2H 2 O+2CO 2 ;
[0053] Detect the pH value every 3 hours of reaction, and when it is detected that the pH value of the solution in the ...
Embodiment 3
[0058] Embodiment three, the present invention is described in detail to producing potassium thiosulfate and reclaiming carbon dioxide as example:
[0059] Such as figure 1 Shown, the present invention utilizes industrial flue gas to produce potassium thiosulfate and the method for producing pure carbon dioxide, comprises the following steps:
[0060] 1. The KHCO 3 Prepare an absorption liquid with a concentration of 16.2% and a pH value of 9.6, fully contact the flue gas containing sulfur dioxide with the absorption liquid in the absorption tower, control the temperature of the flue gas entering the absorption tower at 100°C, and control the concentration of sulfur dioxide at 2000ppm. The sulfur dioxide in the flue gas is dissolved in the absorption liquid, and the absorption reaction formula is:
[0061] 2KHCO 3 +SO 2 +H 2 O=K 2 SO 3 +2H 2 O+2CO 2 ;
[0062] The pH value is detected once every 3 hours of reaction, and when the pH value of the solution in the absorp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com