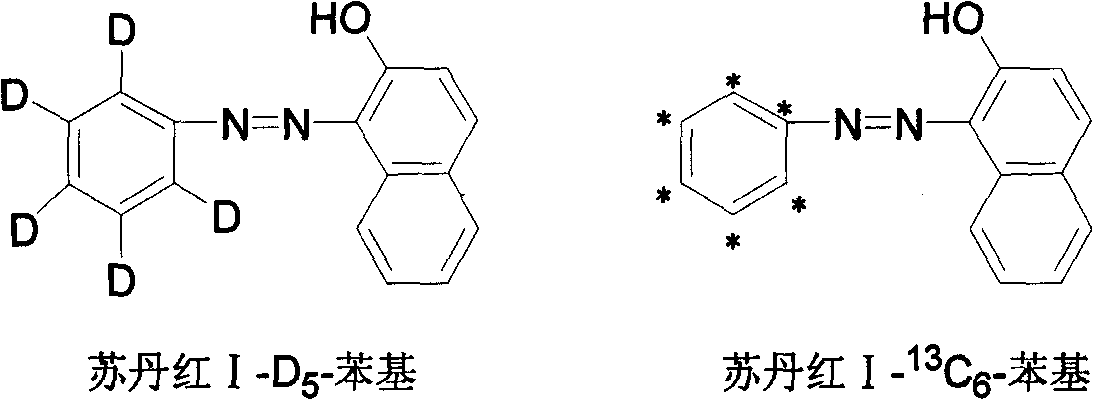
Stable isotope labeled sudan red I and its synthesis method
A technology of stable isotope and synthesis method, which is applied in the field of stable isotope labeling of Sudan Red I and its synthesis, can solve the problems of high price of deuterated aniline, and it is difficult to meet the requirements of detection reagents, and achieves good economy, use value, and atom utilization. High rate, simple process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
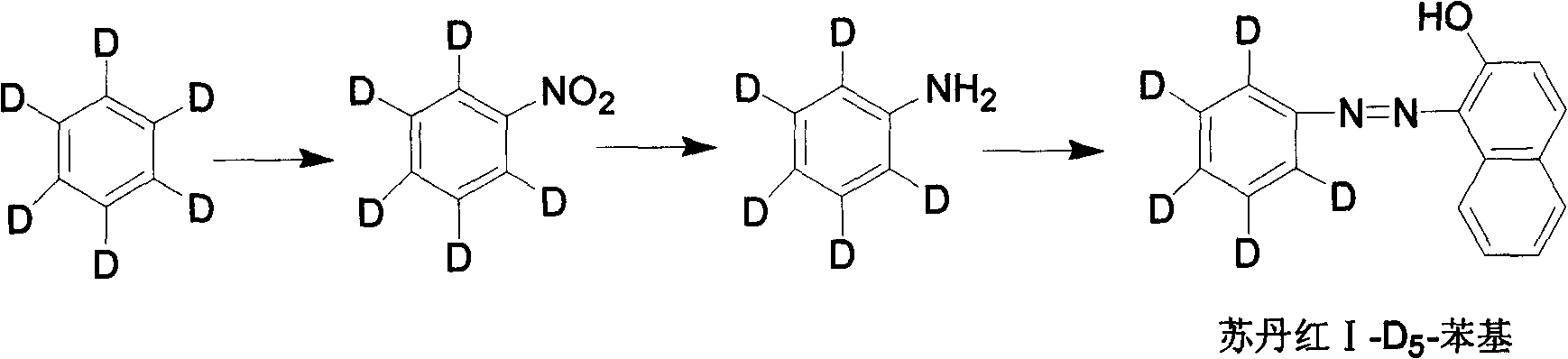
[0029] A stable isotope labeled Sudan Red I-D 5 A method for preparing phenyl, the method comprising the following steps:
[0030] 1. Stable isotope labeled nitrobenzene-D 5 preparation of
[0031] In a 100mL three-neck flask, add benzene-D 5 8.4g, add a mixture of 35g concentrated nitric acid and concentrated sulfuric acid dropwise at room temperature, raise the temperature to 40°C after the dropwise addition, react for 2 hours, separate and purify to obtain light yellow nitrobenzene-D 5 10.5g, yield 82.0%, GC detection, purity ≥ 98%; mass spectrometry detection, abundance ≥ 98atom%D.
[0032] 2. Stable isotope labeled aniline-D 5 preparation of
[0033] Add nitrobenzene-D into a 100mL three-necked flask 5 6.4g, add 3ml of concentrated hydrochloric acid, 10ml of water, 13g of iron powder, heat up to 40°C, react for 5 hours, separate and purify to obtain light yellow aniline-D 5 4.5g, yield 91.8%, GC detection, purity ≥ 98%; mass spectrometry detection, abundance ≥ 98at...
Embodiment 2
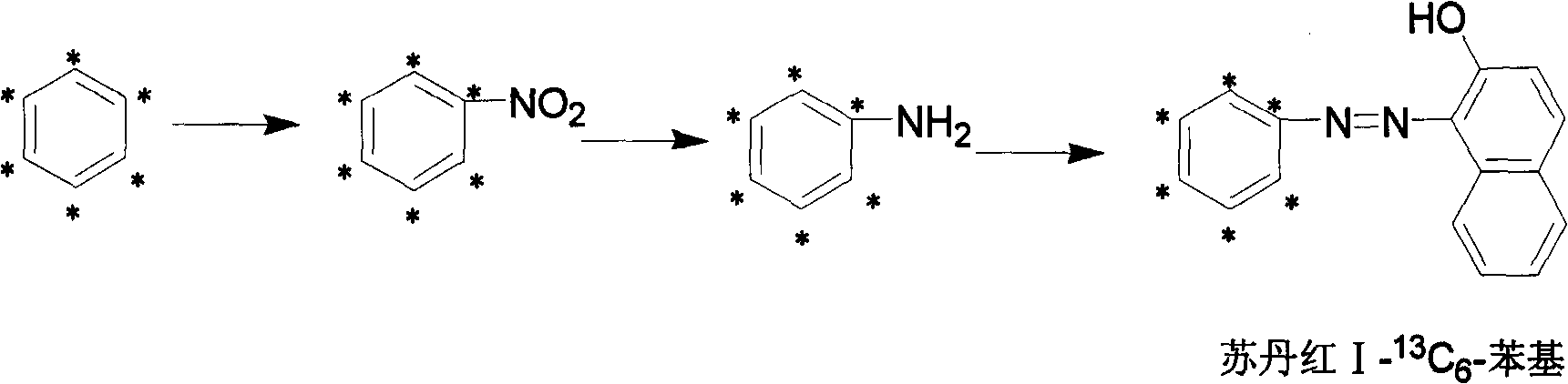
[0038] A stable isotope labeled Sudan red I- 13 C 6 A method for preparing phenyl, the method comprising the following steps:
[0039] 1. Stable isotope labeled nitrobenzene- 13 C 6 preparation of
[0040] Add benzene- 13 C 6 4.2g, add 30g of concentrated nitric acid and concentrated sulfuric acid mixture dropwise at 40°C, heat up to 60°C after the dropwise addition, react for 1 hour, separate and purify to obtain light yellow nitrobenzene- 13 C 6 5.4g, yield 84.4%, GC detection, purity ≥ 98%; mass spectrometry detection, abundance ≥ 98atom% 13 c.
[0041] 2. Stable isotope labeled benzene- 13 C 6 preparation of
[0042] Add nitrobenzene- 13 C 66.45g, 30ml of tetrahydrofuran, 10g of sodium borohydride was added in batches at 0°C, and after the addition was completed, the temperature was raised to 20°C, and reacted for 5 hours, separated and purified to obtain light yellow aniline- 13 C 6 4.6g, yield 92.9%, GC detection, purity ≥ 98%; mass spectrometry detection,...
Embodiment 3
[0047] A kind of synthetic method of stable isotope label Sudan red I, this method comprises the following steps:
[0048] (1) The stable isotope labeled benzene and nitric acid with a molar ratio of 1:1 are reacted under the action of catalyst sulfuric acid to prepare stable isotope labeled nitrobenzene, and the reaction temperature is 0°C;
[0049] (2) Stable isotope labeled nitrobenzene in solvent water and reducing agent H 2 The reaction generates stable isotope-labeled aniline, the molar ratio of the stable isotope-labeled nitrobenzene to the reducing agent is 1:1, and the reaction temperature is 0°C;
[0050] (3) Stable isotope-labeled aniline, diazotized under acidic conditions of acetic acid, and then coupled with naphthol to obtain stable isotope-labeled Sudan I; the molar ratio of the stable isotope-labeled aniline to naphthol is 1: 1, The temperature of the coupling reaction is 0°C.
[0051] Stable isotope labeled benzene can be stable isotope 13 C label or stabl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com