Method for extracting and separating lithium isotope aqueous solution
A lithium isotope and aqueous solution technology, applied in the field of solvent extraction, can solve the problems of high production cost and serious environmental pollution, and achieve the effects of saving production cost, improving isotope extraction rate, and significant isotope separation ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
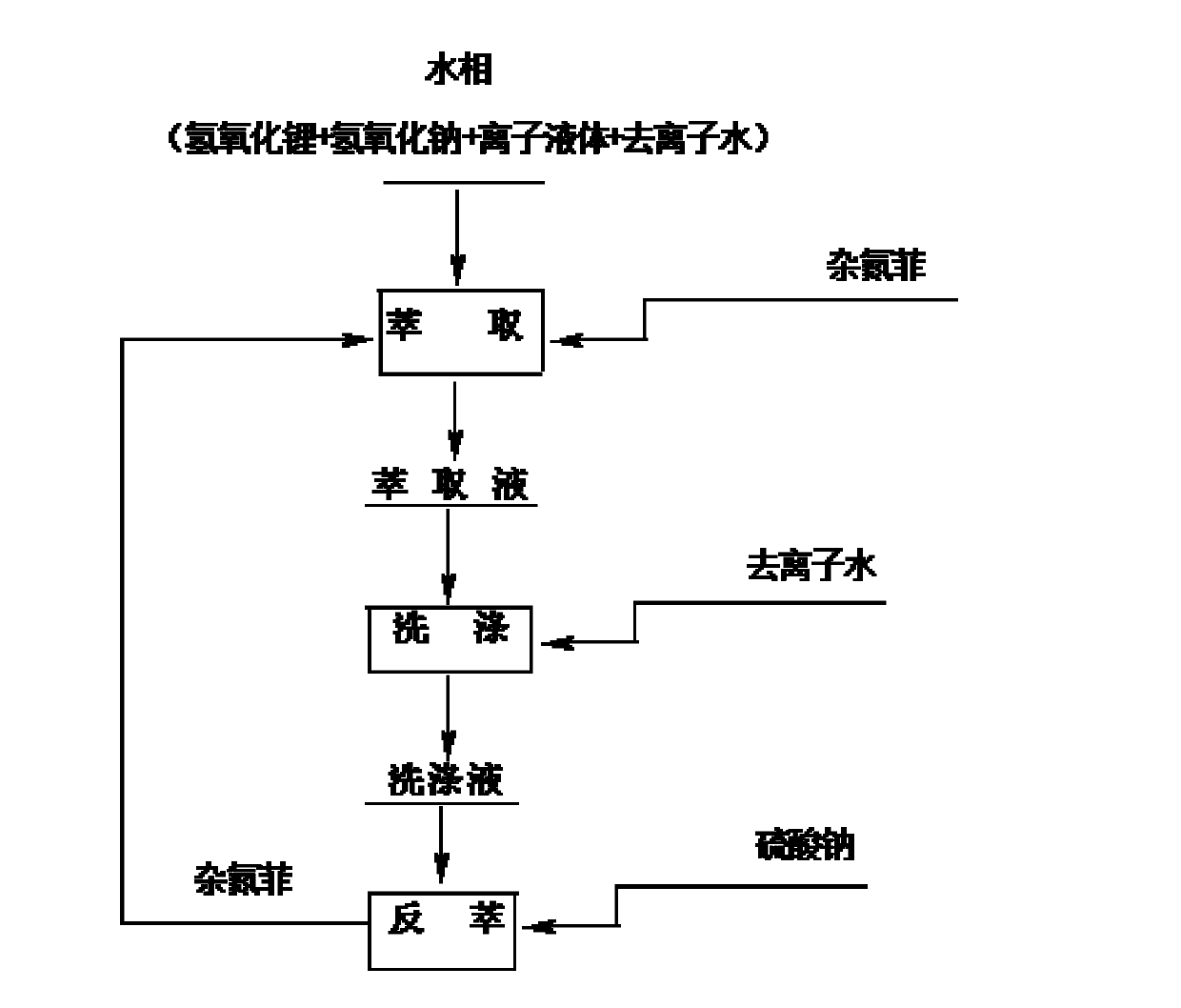
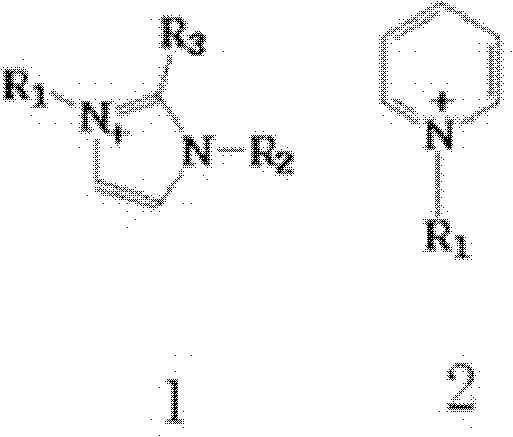
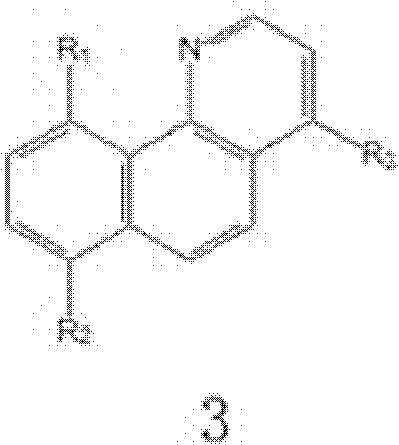
[0032]In a 250mL separatory funnel, add 20mL of water phase (0.1mol / L lithium hydroxide+1.6mol / L sodium hydroxide+0.2mol / L 1-butyl-3-methylimidazolium bromide, in which lithium ion: Hydroxide ion: the ratio of the molar concentration of co-extractant is 1:17:2) and 40mL organic phase (1,2-dichlorobenzene solution of 0.2mol / L azaphenanthrene), shake vigorously for about 20 minutes, centrifuge The aqueous and organic phases were separated and the organic phase was collected. After the organic phase was washed once with 5 mL deionized water, 0.1 mol / L Na 2 SO 4 The solution was 20 mL, shaken vigorously for about 20 minutes, centrifuged, and the aqueous phase was collected. The organic phase was directly used for the next extraction, and the operation was repeated fifteen times. Once / fifteen times lithium extraction rate is 15.2% / 99.3%, once / fifteen times lithium stripping rate is 12%, 99.1%, isotope separation coefficient ( 7 Li / 6 Li) was 1.022.
Embodiment 2
[0034] In a 250mL separatory funnel, add 20mL of water phase (0.1mol / L lithium hydroxide + 1.6mol / L sodium hydroxide + 0.2mol / L 1-octyl-3-methylimidazole bromide, in which lithium ion: Hydroxide ion: the ratio of the molar concentration of co-extractant is 1:17:2) and 20mL organic phase (1,2-dichlorobenzene solution of 0.2mol / L azaphenanthrene), shake vigorously for about 30 minutes, centrifuge The aqueous and organic phases were separated and the organic phase was collected. After the organic phase was washed once with 5 mL deionized water, 0.1 mol / L Na 2 SO 4 40mL of the solution was shaken vigorously for about 30 minutes, centrifuged, and the aqueous phase was collected. The organic phase was directly used for the next extraction, and the operation was repeated fifteen times. Once / fifteen times lithium extraction rate is 16.6% / 99.7%, the stripping rate of once / fifteen times lithium is 12.7% / 99.5%, isotope separation coefficient ( 7 Li / 6 Li) was 1.024.
Embodiment 3
[0036] In a 250mL separatory funnel, add 40mL of water phase (0.2mol / L lithium hydroxide + 4mol / L sodium hydroxide + 0.2mol / L 1-octyl-3-methylimidazolium tetrafluoroborate, in which lithium ions : hydroxide ion: the molar concentration ratio of co-extractant is 1: 20: 1) and 20mL organic phase (1,3-dichlorobenzene solution of 0.2mol / L azaphenanthrene), shake vigorously for about 50 minutes, The aqueous and organic phases were separated by centrifugation, and the organic phase was collected. After the organic phase was washed once with 10 mL deionized water, 0.5 mol / L Na 2 SO 4 40mL of the solution was shaken vigorously for about 50 minutes, centrifuged, and the aqueous phase was collected. The organic phase was directly used for the next extraction, and the operation was repeated fifteen times. Once / fifteen times lithium extraction rate is 17.1% / 99.9%, once / fifteen times lithium stripping rate is 16.0% / 99.6%, isotope separation coefficient ( 7 Li / 6 Li) is 1.020.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com