Method for preparing spherical Bayer stones
A Bayerite, spherical technology, applied in the field of preparing spherical Bayerite, can solve the problems of irregular shape of Bayerite, high cost of alumina, uneven particle size, etc., achieve shortened decomposition time, low content of alkaline ions and sodium ions, and particle size The effect of uniform diameter
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
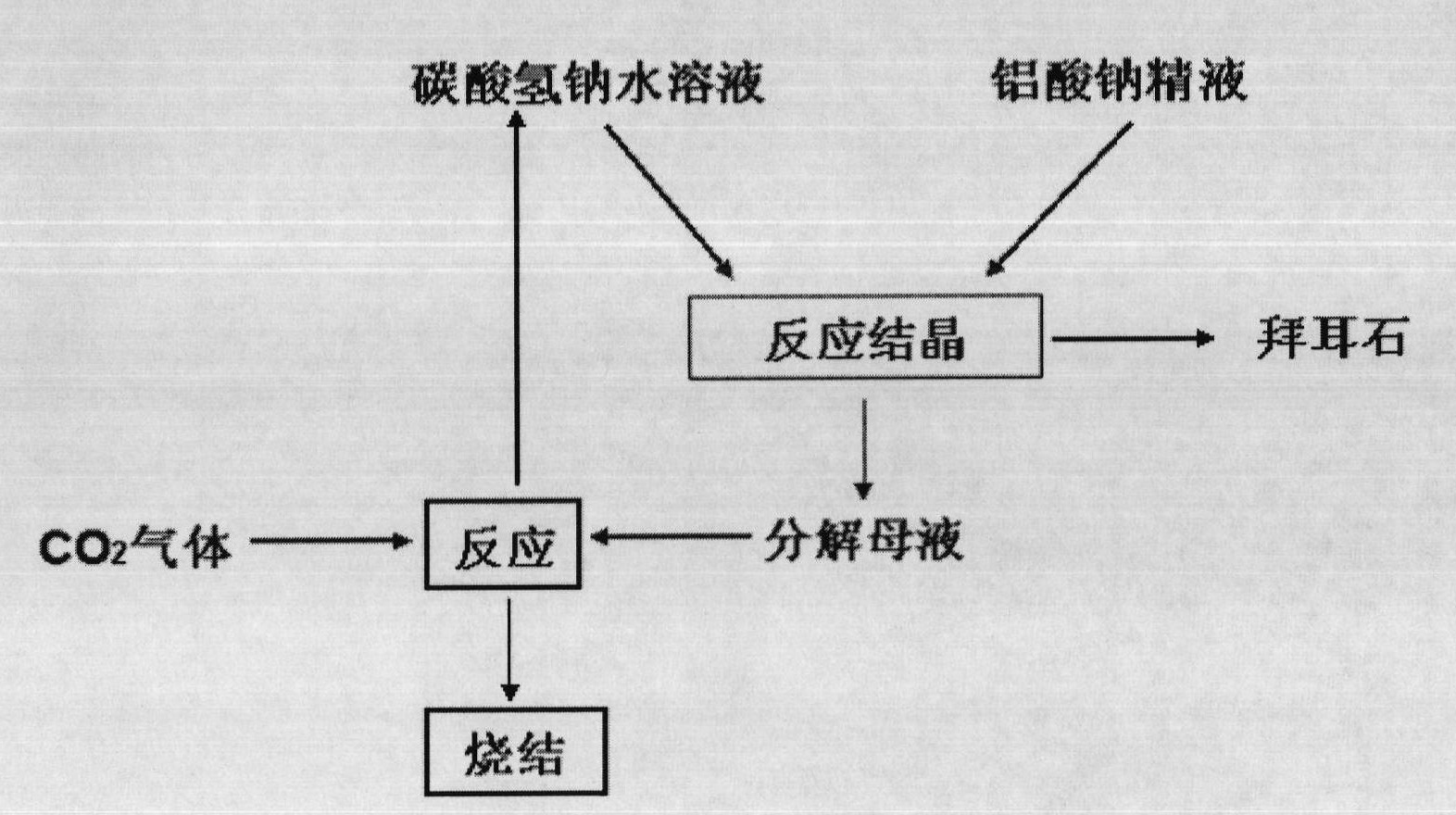
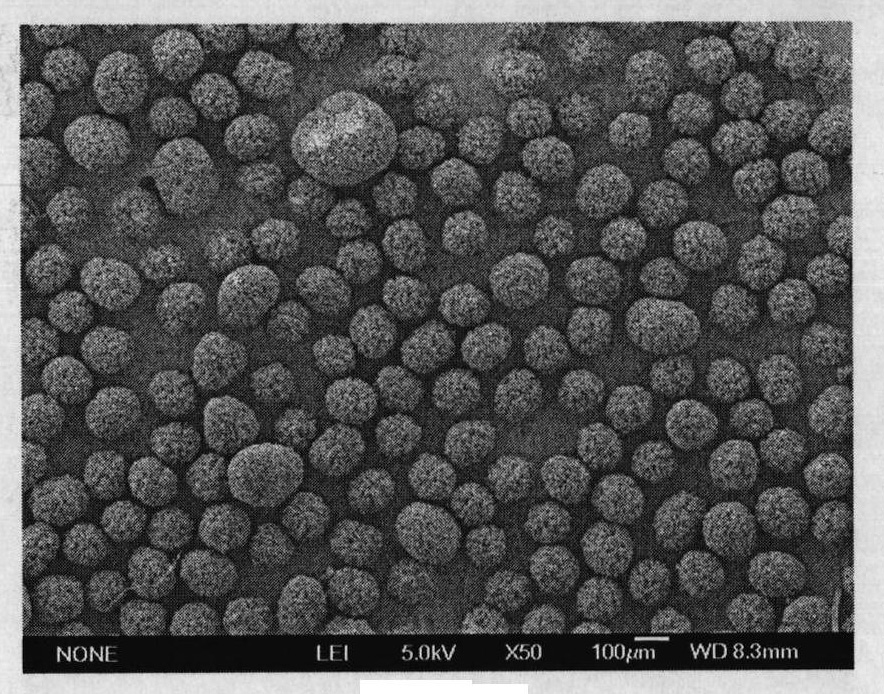
[0043] The sodium aluminate semen after desilication in industrial alumina production is evaporated to the composition: Na 2 O 150g / L, Al 2 o 3 164.5g / L, molecular ratio 1.5. Dissolve 150 g of sodium bicarbonate in 3000 mL of deionized water to obtain a 50 g / L aqueous solution of sodium bicarbonate. Add the aqueous sodium bicarbonate solution and sodium aluminate solution continuously at a rate of 4.61 mL / min and 1.12 mL / min for continuous crystallization In the reactor, the molar ratio of sodium bicarbonate to sodium aluminate is 1.01:1. The reaction process is carried out in a constant temperature water bath at 50°C, with an average residence time of 90min. After the reaction reaches a steady state, the solid-liquid mixture is carried out. Separate, wash the solid phase with hot water until the washing solution is neutral, and dry at 100°C for 24 hours to obtain Bayerite products, such as figure 2 As shown, the obtained product is a regular spherical shape with uniform p...
Embodiment 2
[0045] Mix 50g / L aqueous sodium bicarbonate solution and sodium aluminate solution (Na 2 O 150g / L, Al 2 o 3 164.5g / L, molecular ratio 1.5) was dripped continuously in the continuous reaction crystallizer with the speed of 4.26mL / min and 1.17mL / min respectively, the molar ratio of sodium oxide in sodium bicarbonate and sodium aluminate is 0.90:1, The process is carried out in a constant temperature water bath at 40°C, with an average residence time of 90 minutes. After the reaction reaches a steady state, the solid-liquid mixture is separated, and the solid phase is washed with hot water until the washing liquid is neutral, and dried at 100°C for 24 hours, namely To obtain spherical Bayerite products, such as image 3 As shown, the obtained product is a regular spherical shape with uniform particle size distribution. The decomposition mother liquor reacts with carbon dioxide gas to obtain sodium bicarbonate solution again.
Embodiment 3
[0047] Mix 100g / L aqueous sodium bicarbonate solution and sodium aluminate solution (Na 2 O 200g / L, Al 2 o 3 164.5g / L, molecular ratio 2.0) is dripped continuously in the continuous crystallization reactor with the speed of 4.38mL / min and 1.16mL / min respectively, and the mol ratio of sodium oxide in sodium bicarbonate and sodium aluminate is 1.39: 1, The process is carried out in a constant temperature water bath at 50°C, with an average residence time of 90 minutes. After the reaction reaches a steady state, the solid-liquid mixture is separated, and the solid phase is washed with hot water until the washing liquid is neutral, and dried at 100°C for 24 hours, namely To obtain spherical Bayerite products, such as Figure 4 As shown, the obtained product is a regular spherical shape with uniform particle size distribution. The decomposition mother liquor reacts with carbon dioxide gas to obtain sodium bicarbonate solution again.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com