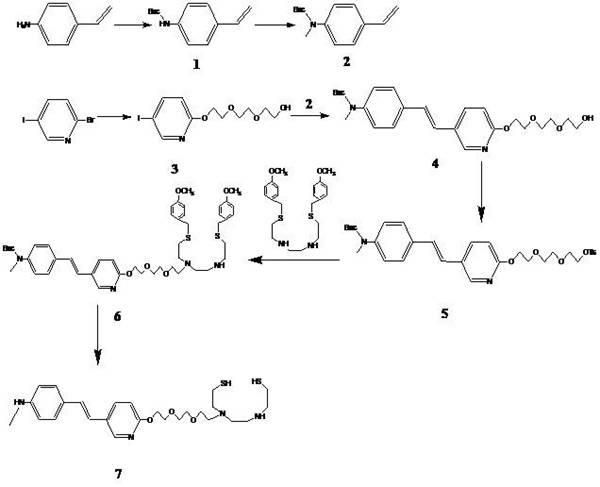
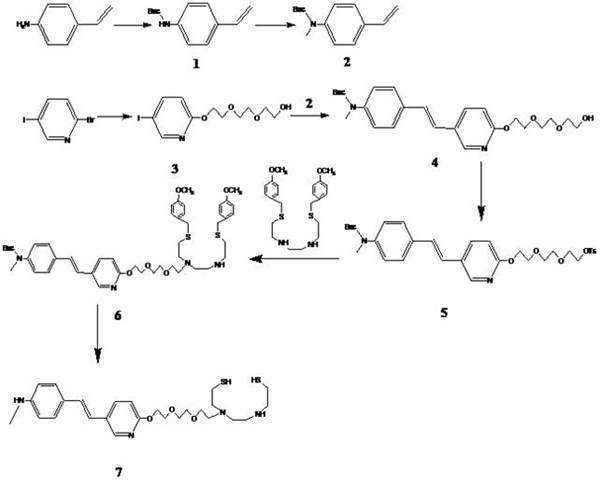
Styryl pyridine disulfide dinitrogen derivative and preparation method thereof
A technology of methylaminostyrene and tert-butoxycarbonylmethylaminostyrene, which is applied in the field of synthesis of radiopharmaceutical-labeled precursor compounds, and can solve problems such as no relevant reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthesis of tert-butyl 4-vinylphenylcarbamate (compound 1)
[0034] 4-Aminostyrene and di-tert-butylmethyl dicarbonate with 1.1 times the molar weight of 4-aminostyrene were dissolved in an appropriate amount of water, stirred at room temperature for 3 hours, a solid was formed, the solid was filtered out, dissolved in ethyl acetate, and the aqueous phase was washed with acetic acid Extract with ethyl ester, combine the organic phases, wash with saturated aqueous sodium chloride solution, dry over anhydrous sodium sulfate, and remove the solvent under reduced pressure to obtain light yellow solid compound 1, namely tert-butyl 4-vinylphenylcarbamate, which is directly used in Next reaction.
Embodiment 2
[0036] Synthesis of tert-butyl N-methyl-4-vinylphenylcarbamate (compound 2)
[0037] N 2 For protection, dissolve 6.7g (30mmol) of compound 1 in 30mL of anhydrous DMF, cool in an ice bath to about 0°C, slowly add 1.8g (45mmol) of 60% sodium hydride in 100mL of anhydrous DMF solution dropwise while stirring, and drop , raised to room temperature, 8.5g (60mmol) methyl iodide was slowly added dropwise to the reaction mixture, continued to stir for 2 hours, the reaction solution was carefully added to 200mL ice water, extracted with ethyl acetate (70mL×3 times), the ester layer Washed with saturated aqueous sodium chloride, dried over anhydrous sodium sulfate, and removed the solvent under reduced pressure to obtain red oil 2 (6.5 g, 89%), which was directly used in the next reaction.
Embodiment 3
[0039] Synthesis of 2-(2-(2-(5-iodopyridine-2-oxyl)ethoxy)ethoxy)ethanol (compound 3)
[0040] Dissolve 5g (17.6mmol) of 2-bromo-5-iodopyridine, 5.3g (70mmol) of triethylene glycol in 50mL of tetrahydrofuran, and add 2.35g (19mmol) of potassium tert-butoxide into the reaction solution in batches under stirring at room temperature. Reflux reaction for 24 hours, remove tetrahydrofuran under reduced pressure, add ethyl acetate and water, separate liquids, extract the aqueous phase with ethyl acetate, combine organic phases, wash with saturated aqueous sodium chloride solution, dry over anhydrous sodium sulfate, remove under reduced pressure solvent to obtain a pale yellow oil, which solidified at room temperature and was separated by ethyl acetate column chromatography to obtain product 3 (2.34 g, 75%). [ 1 H] NMR (500MHz, CDCl 3 ) δ: 8.30 (1H, d), 7.76 (1H, d, d), 6.63 (1H, d), 4.44 (2H, t), 3.84 (2H, t), 3.73-3.60 (6H, m), 3.61 (2H,t).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com