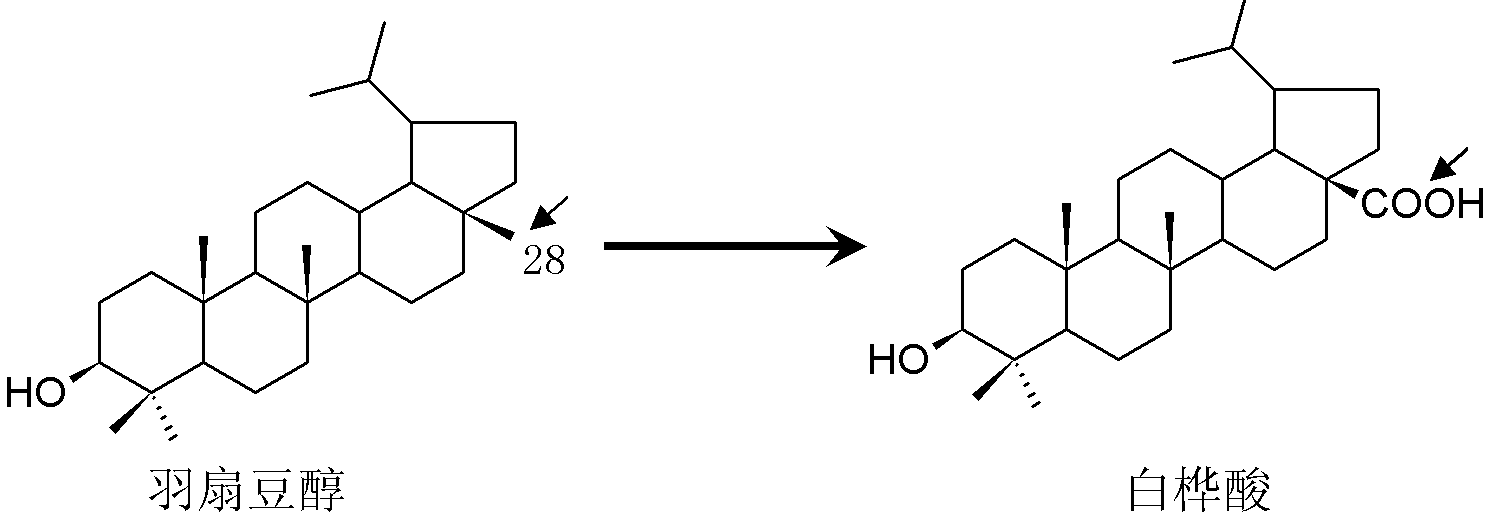
Lupeol C28 oxidase gene and preparation method and application thereof
A lupeol and oxidase technology, applied in biochemical equipment and methods, applications, genetic engineering, etc., can solve problems such as destruction of plant resources, high production costs, and unreported P450 genes, and achieve great application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0035] Below in conjunction with accompanying drawing and embodiment describe in detail:
[0036] 1. Acquisition of the full-length cDNA sequence of the lupeol C28 oxidase gene
[0037] According to GenBank ( www.ncbi.nlm.nih.gov ) provided in the 3 sequence expression tags (the registration numbers of the sequence tags in GenBank are FD660831, FD661068 and FD661229) information, use RACE-PCR technology to obtain the full-length cDNA sequence information of this gene, and then use the following general PCR The reaction system can be obtained.
[0038] 25 μl PCR reaction system: 2 μl cDNA template, 1 μl each of primers P1 and P2, 5 μl 5×Buffer, 2 μl dNTP Mixture (2.5 mmol / L each), 0.2 μl Phusion enzyme (NEB Biotechnology Co., Ltd.), ddH 2 O 13.8 μl;
[0039] The reaction conditions were as follows: pre-denaturation at 98°C for 3 minutes; then 35 cycles at 98°C for 10s, 62°C for 30s, and 72°C for 45s; finally, extension at 72°C for 5 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com