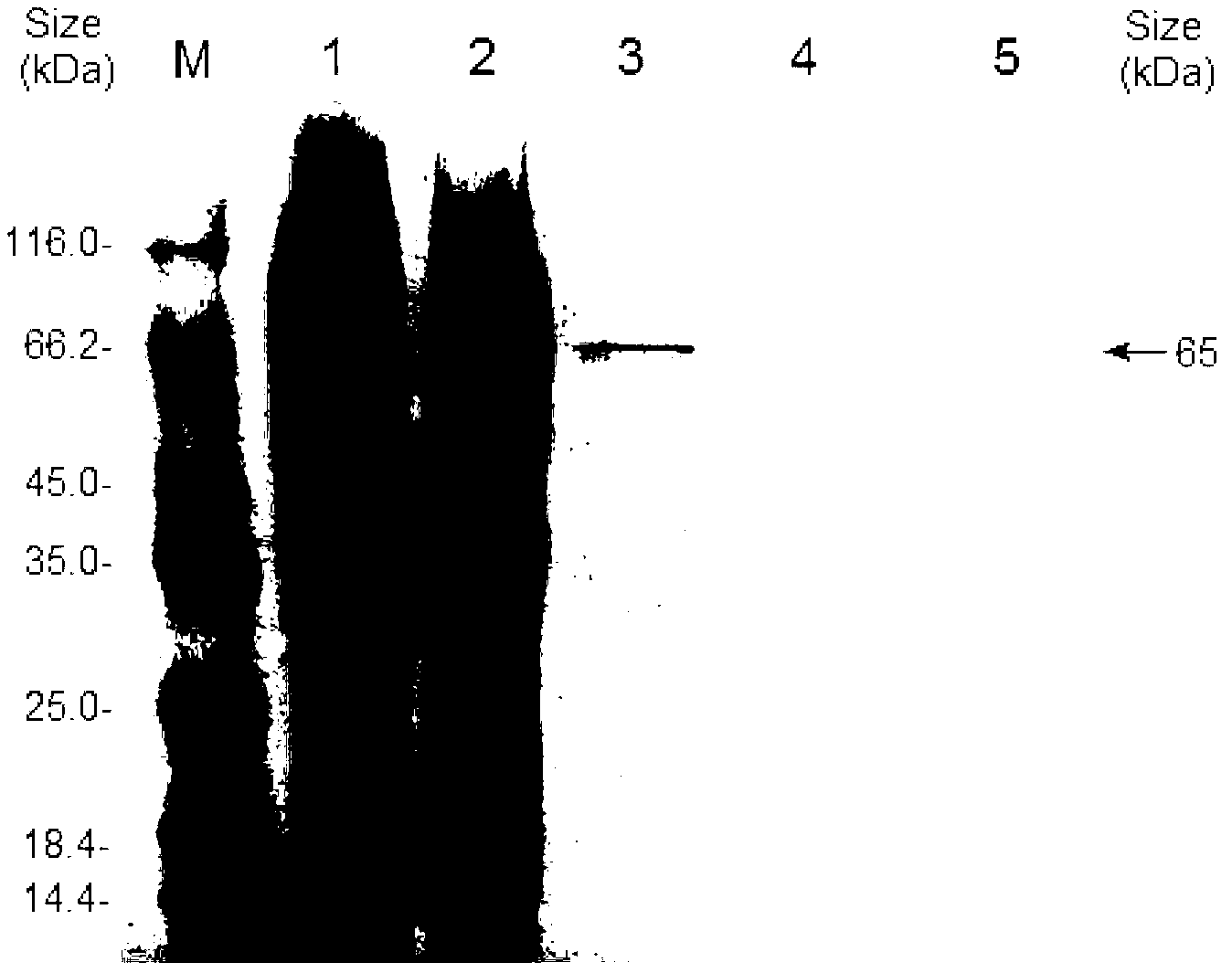Bacillus pumilus laccase gene as well as expression and application thereof
A technology of Bacillus pumilus and laccase, applied in application, genetic engineering, plant genetic improvement, etc., can solve the problems of secondary pollution, poor effect, etc., and achieve high yield, good tolerance, and high temperature resistance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
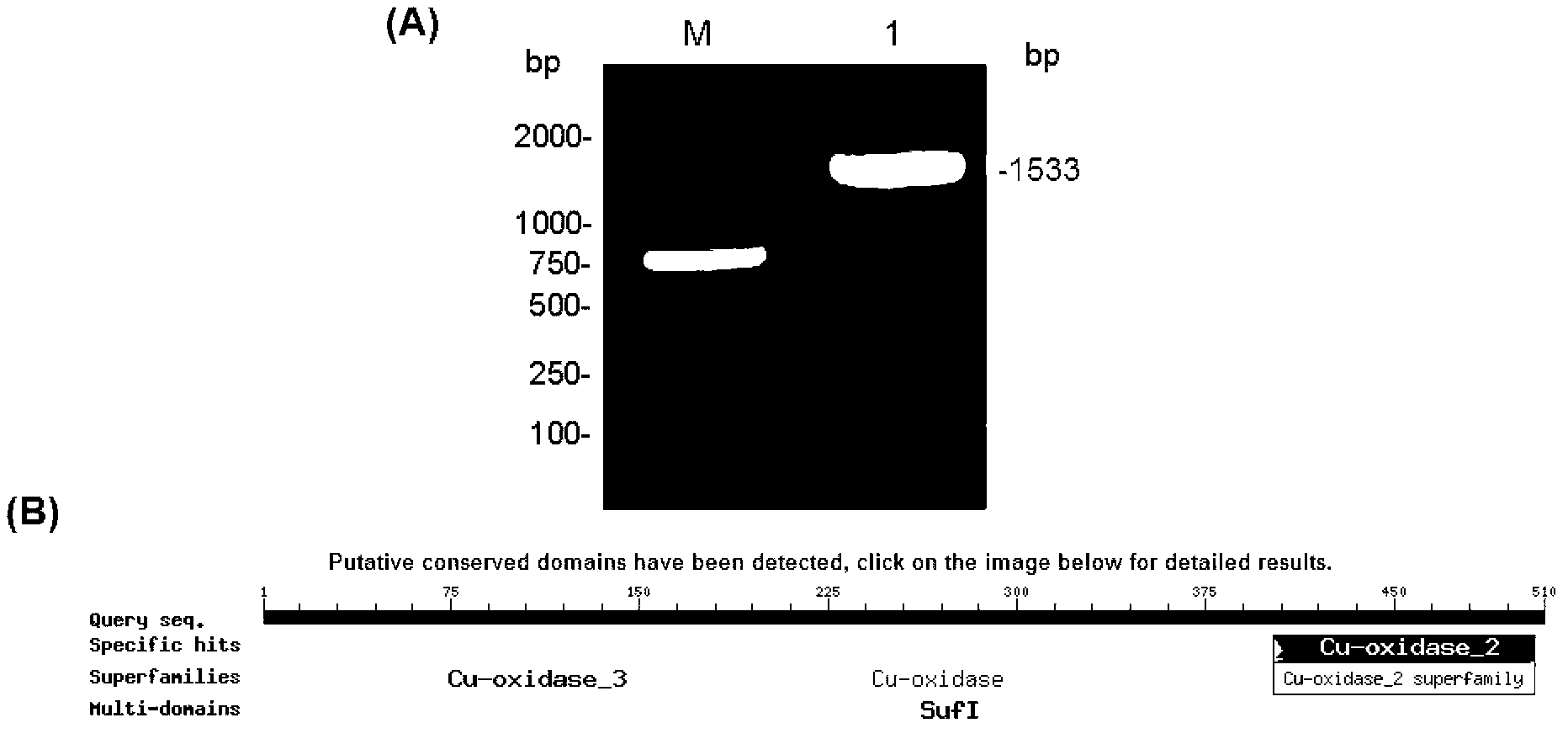
[0033] Example 1 PCR amplification of B. pumilus L-9 laccase gene cotA, protein sequence functional region prediction
[0034] Bacterial Genomic DNA Extraction Kit (TaKaRa Company) was used to extract the total genomic DNA of B. pumilus L-9 bacteria according to its operation manual, its quality and purity were identified by agarose gel electrophoresis, and its concentration was determined by ultraviolet spectrophotometer. The upstream and downstream primers were designed according to the laccase gene sequences of other Bacillus pumilus strains published in the GenBank database: g3 (SEQ ID NO: 3, 5′-ATGAACCTAGAAAAATTTGTTGACGAGCTG-3′) and g4 (SEQ ID NO: 4, 5′- TTACTGGATGATATCCATCGGCCGCATC-3'), using the PCR method, using the above-mentioned B. pumilus L-9 genome as a template, and using the above-mentioned g3 and g4 as specific primers, the full-length coding frame sequence of the B. pumilus L-9 laccase gene was amplified ( 1533bp) (eg figure 1 Shown in A), then use the T-A c...
Embodiment 2
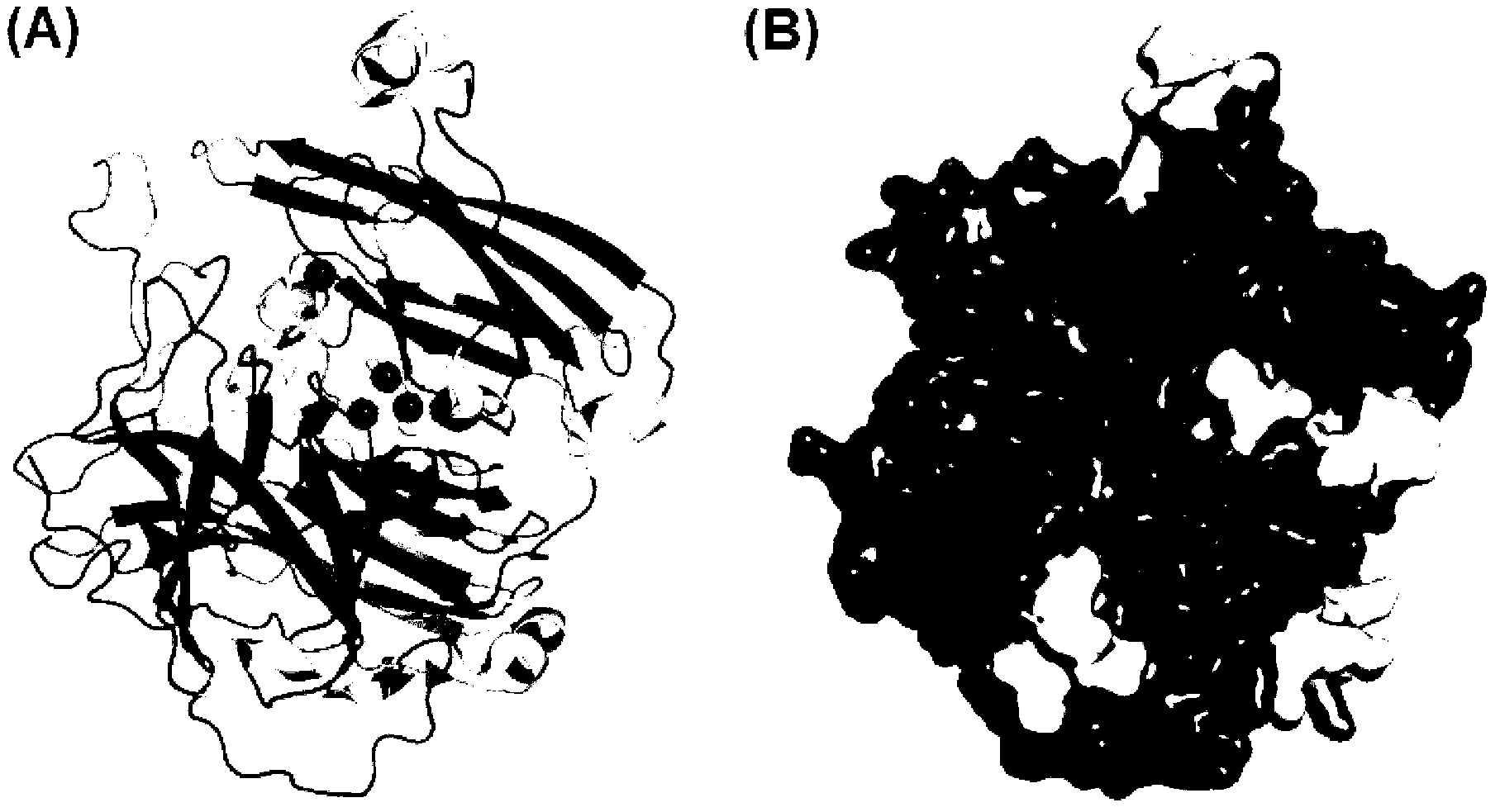
[0035] Example 2 Obtaining the Predicted Crystal Structure of B. pumilus L-9 Laccase Using "Homologous Modeling" Method
[0036] Submit the amino acid sequence of B. pumilus L-9 laccase to the SWISS-MODEL protein online modeling server (http: / / swissmodel.expasy.org / ) for homology modeling, and the optimal modeling template obtained by automatic analysis of the server is The crystal structure of B. subtilis MB24 laccase I494A mutant (PDB file: 2wsdA) was subsequently shown using the RasWin Molecular Graphics Program (RasMol, 156version2.7.2) software package (http: / / www.umass.edu / microbio / rasmol / ) The predicted structure of B.pumilus L-9 laccase crystal shown by Figure software is as follows figure 2 shown.
Embodiment 3B
[0037] Escherichia coli recombinant expression, purification, activity staining and immunoblotting identification of embodiment 3B.pumilus L-9 laccase
[0038] Since the laccase protein CotA of the wild strain of B. pumilus is only expressed on the exine wall of the spore and not secreted outside the cell, and the expression level is generally very low, which is far from meeting the requirements of industrial applications, the recombinant expression system of Escherichia coli is used to produce laccase It can greatly increase its output, making it easier to meet the requirements of industrial application. Design primer g5: (SEQ ID NO:5, 5'-TCAGGA GGTACC ATGAACCTAGAAAAATTTGTTG-3′) and g6: (SEQ ID NO:6, 5′-ACTGAG GAATTC TTACTGGATGATATCCATCG-3′), the 5′ and 3′ sides of the DNA coding frame of B. pumilus L-9 laccase were introduced into Kpn1 and EcoRI restriction enzyme sites (shown underlined) respectively by PCR. Ligation and other genetic operations Insert the laccase gene i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com