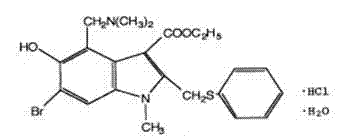
Application of arbidol hydrochloride in preparation of anti-enterovirus medicines
A technology of arbidol hydrochloride and enterovirus, which is applied in antiviral agents, pharmaceutical formulas, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] The toxic effect of embodiment 1 Arbidol hydrochloride on RD cells
[0015] (1) According to Mosmann, the MTT method was established to detect the cytotoxicity of drugs.
[0016] Disperse the well-grown cells into a single cell suspension with trypsin containing 0.02% EDTA, and then adjust the cells to 1×10 with cell culture medium containing 10% calf serum 5 / ml concentration in 96-well plate, 0.2ml per well. Set at 37°C, 5% CO 2 Cultivate in the incubator for 24h. After the cells grow into a single layer, discard the supernatant of the culture medium, and replace with a maintenance solution containing different concentrations of drugs, 200 μl per well. The final concentrations of the drugs were 62.50, 31.25, 15.63, 7.81, 3.91 μg / ml, respectively. Four wells were repeated for each concentration, and a normal cell control group was set up for the experiment. After continuing to culture for 48 h, the cell viability was detected by MTT assay. Results: The toxic ef...
Embodiment 2
[0017] Example 2 Pharmacodynamic study of Arbidol hydrochloride against enterovirus type 71
[0018] 1. The direct killing effect of Arbidol hydrochloride on enterovirus 71
[0019] 50TCID 50 EV71 was mixed with different concentrations of Arbidol hydrochloride solution, the final concentrations were 8, 4, 2, 1, 0.5 μg / ml, respectively, added to the RD cells that had grown into a monolayer at 4 °C for 6 hours, and after 2 hours of adsorption , discard the supernatant, add cell maintenance solution, 37 ° C, 5% CO 2 Continue to grow in the incubator. CPE was observed daily, and when the virus control lesions reached +++~++++, the virus inhibition rate was detected by MTT method. The therapeutic index was used as an evaluation index to measure the inhibitory effect of the drug on the virus. A virus control and a cell control were also set up. Taking TI as the evaluation index of the drug's inhibitory effect on EV71, TI=TC 50 / IC 50 , the larger the TI value, the stronge...
Embodiment 3
[0024] Example 3: Experimental research on anti-EV71 of Arbidol in vivo
[0025] 40 female BALB / c mice (7-9 g) were randomly divided into 4 groups: ① normal animal control group, fed with normal saline; ② virus infection model group, fed with normal saline after virus infection; ③ high-dose treatment group (0.1g·kg -1 d -1 ); ④ low dose treatment group (0.05g·kg -1 d -1 ).
[0026] Mice were anesthetized with ether and treated with 15LD 50The virus liquid was orally infected, each 0.05 mL, and the normal animal control group was orally infected with normal saline instead of the virus liquid. Two hours after infection, intragastric administration was started, administered twice a day, 0.1 mL each time, and administered continuously for 5 days. Observe for 15 days. The mice were fasted for 8 hours before being sacrificed, and the animals were sacrificed by removing the eyeballs, bloodletting, and dismounting the cervical vertebrae. The intestinal tissue was taken out an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com