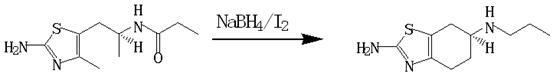Industrial preparation method for pramipexole and its dihydrochloride monohydrate
A technology of amino and tetrahydro, applied in the direction of organic chemistry, can solve problems such as harsh reaction conditions, difficult preparation, damage to reaction equipment, etc., and achieve the effects of mild reaction conditions, high reaction safety, and safe reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Preparation of (S)-(-)2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole
[0034] Add (-)2-amino-6-propionylamino-4,5,6,7-tetrahydrobenzothiazole (227.33g, 1mol) into tetrahydrofuran (1362ml), stir to dissolve, then add boron quickly Sodium hydride (94.68g, 2.5mol) was stirred, then cooled to -5°C, and then slowly added dropwise at -5 to 0°C 2 (253.8g, 1mol) and tetrahydrofuran 725ml (I 2 The mass concentration of the solution is 35%). After dropping, slowly raise the temperature to 35°C, keep the temperature for 8 hours, then cool to below 10°C, first add 45ml of tap water dropwise to avoid excessive reaction, and then add dropwise the 946.8ml of hydrochloric acid, then slowly raise the temperature to 40°C, keep it for 30min, then recover tetrahydrofuran by vacuum distillation, after recovery, adjust the pH of the remaining solution to 12 with 30% sodium hydroxide solution, precipitate a large amount of solids, cool down to below 10°C , stirred for 30 minutes...
Embodiment 2
[0038] (1) Preparation of (S)-(-)2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole
[0039] Add (-)2-amino-6-propionylamino-4,5,6,7-tetrahydrobenzothiazole (227.33g, 1mol) into tetrahydrofuran (2270ml), stir to dissolve, and then quickly add boron Sodium hydride (37.83g, 1mol) was stirred, then cooled to -30°C, and then slowly added dropwise at -30~-5°C 2 (101.52g, 0.40mol) and tetrahydrofuran 253.8ml (I 2 The mass concentration is 40%) solution, after dropping, slowly heat up to 40°C, keep warm for 6 hours, then cool to below 10°C, first add 18ml of water dropwise to avoid excessive reaction, then add dropwise hydrochloric acid with a mass fraction of 37% 454ml, then slowly raised the temperature to 40°C, kept for 30min, and then recovered tetrahydrofuran by vacuum distillation. After recovery, the remaining solution was adjusted to pH=12 with 30% sodium hydroxide solution, and a large amount of solids were precipitated, cooled to below 10°C, and stirred 30min, filter ...
Embodiment 3
[0043] (1) Preparation of (S)-(-)2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole
[0044] Add (-)2-amino-6-propionylamino-4,5,6,7-tetrahydrobenzothiazole (227.33g, 1mol) into tetrahydrofuran (1362ml), stir to dissolve, then add boron quickly Sodium hydride (94.68g, 2.5mol) was stirred, then cooled to -30°C, and then slowly added dropwise at -30 to -20°C 2 (253.8g, 1mol) and tetrahydrofuran 725ml (I 2 The mass concentration is 35%) solution, after dropping, slowly heat up to 35°C, keep the temperature for 8 hours, then cool to below 10°C, first add 45ml of water dropwise to avoid excessive reaction, then add dropwise hydrochloric acid with a mass fraction of 37% 946.8ml, then slowly heated up to 40°C, kept for 30min, and then recovered tetrahydrofuran by vacuum distillation. After the recovery, the remaining solution was adjusted to pH = 12 with 30% sodium hydroxide solution, a large amount of solids were precipitated, and the temperature was lowered to below 10°C. Sti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com