Organic luminescent material 4, 6-diphenyl-1, 9-anthralin compounds, synthesis method and application thereof
A synthesis method and compound technology, applied in 4 fields, can solve the problems of affecting performance test, not very good solubility, not very good solubility of anthraline-based small molecule compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
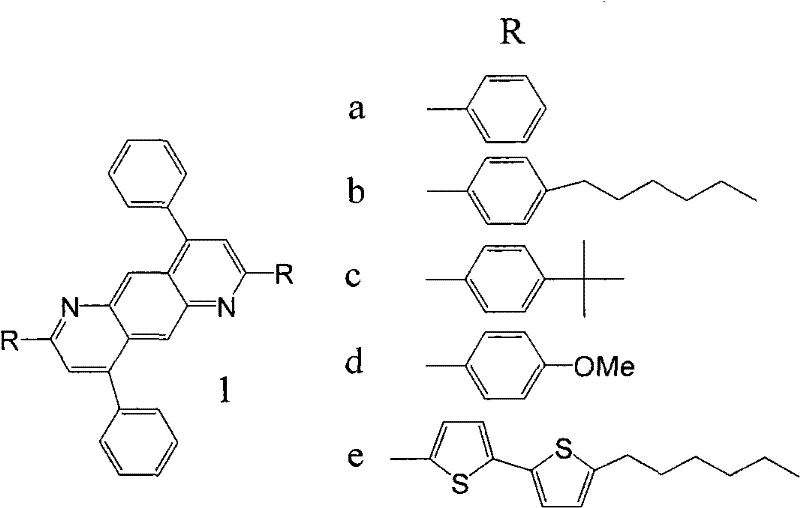
[0045] Embodiment 1: 2,4,6,8-tetraphenyl-1,9-anthracene ( 1a )Synthesis
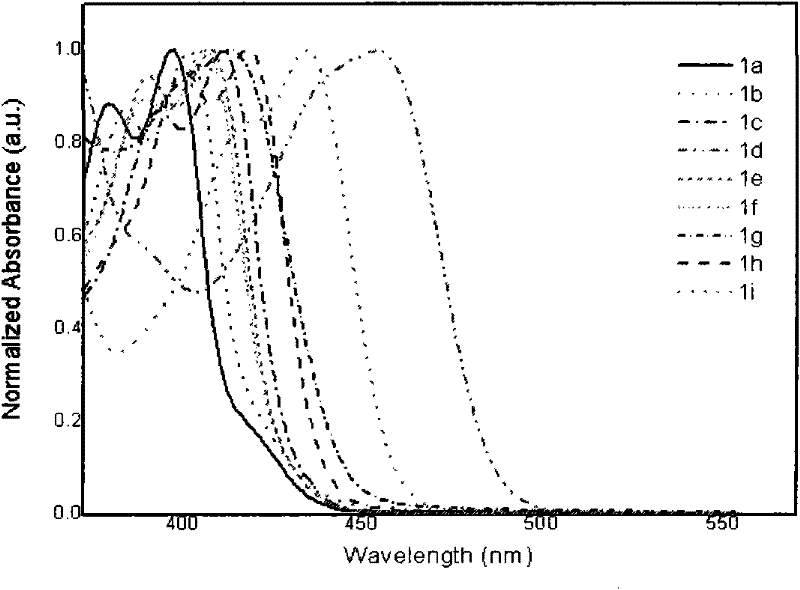
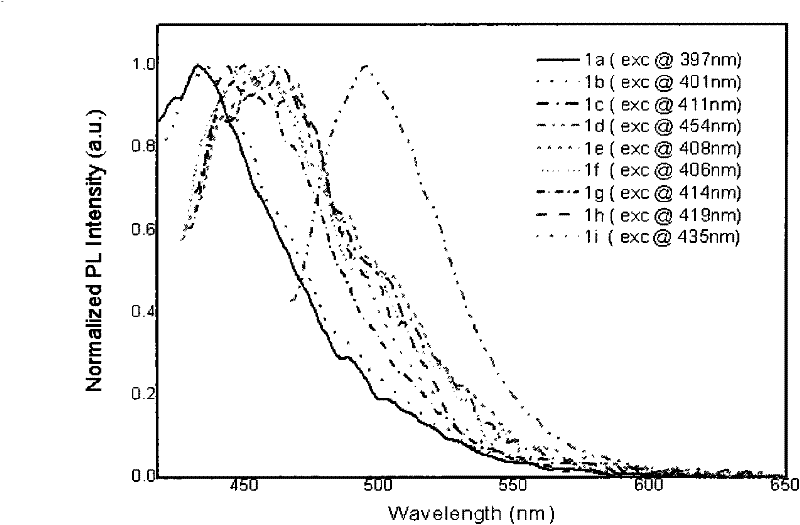
[0046] 4,6-dibenzoyl-1,3-m-phenylenediamine (0.6328g, 2.0mmol), acetophenone 0.5046g (4.2mmol) was added to polyphosphoric acid (4.2g) and m-cresol (10ml) and mixed In the solution, stir magnetically, control the temperature at 130-140°C, and react for 50h. Then cool to 80°C, dilute with methanol (50ml), pour 1mol·L -1 KOH aqueous solution (500ml), filtered, and the precipitate was collected, washed 3 times with hot water (500ml each time), and dried. Finally, recrystallize twice with methanol / THF solution (methanol / THF volume ratio is 30-60%) to obtain orange-yellow flaky solid 2,4,6,8-tetraphenyl-1,9-anthraline (1a ) 0.78g, yield 80.5%, melting point 265.4 ℃, see attached figure 1 , the fluorescence emission spectrum is shown in the attached figure 2 .
Embodiment 2
[0047] Embodiment 2: 2,8-two (p-tert-butylphenyl)-4,6-diphenyl-1,9-anthracene ( 1b )Synthesis
[0048] 4,6-dibenzoyl-1,3-m-phenylenediamine (0.6328g, 2.0mmol), p-tert-butylacetophenone 0.7402g (4.2mmol) was added to polyphosphoric acid (4.2g) and m-cresol (10ml) in the mixed solution, magnetically stirred, temperature controlled at 130-140°C, and reacted for 50h. Then cool to 80°C, dilute with methanol (50ml), pour 1mol·L -1 KOH aqueous solution (500ml), filtered, and the precipitate was collected, washed 3 times with hot water (500ml each time), and dried. Finally, recrystallize twice with methanol / THF solution (methanol / THF volume ratio is 30-60%) to obtain yellow flaky solid 2,8-bis(p-tert-butylphenyl)-4,6-diphenyl -1,9-Anthracene ( 1b ) 0.72g, yield 60.4%, melting point 280.9 ℃, see attached figure 1 , the fluorescence emission spectrum is shown in the attached figure 2 .
Embodiment 3
[0049] Embodiment 3: 2,8-two (p-methoxyphenyl)-4,6-diphenyl-1,9-anthracene ( 1c )Synthesis
[0050] 4,6-dibenzoyl-1,3-m-phenylenediamine (0.6328g, 2.0mmol), p-methoxyacetophenone 0.6303g (4.2mmol) was added to polyphosphoric acid (4.2g) and m-cresol (10ml) in the mixed solution, magnetically stirred, temperature controlled at 130-140°C, and reacted for 50h. Then cool to 80°C, dilute with methanol (50ml), pour 1mol·L -1 KOH aqueous solution (500ml), filtered, and the precipitate was collected, washed 3 times with hot water (500ml each time), and dried. Finally, recrystallize twice with methanol / THF solution (methanol / THF volume ratio is 30-60%) to obtain bright yellow needle-like solid 2,8-bis(p-methoxyphenyl)-4,6-diphenyl Base-1,9-anthracene ( 1c ) 0.70g, yield 64.3%, melting point 266.5 ℃, see attached figure 1 , the fluorescence emission spectrum is shown in the attached figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com