Tazobactam synthesis method
A technology for tazobactam and a synthesis method, which is applied in the field of synthesis of β-lactamase inhibitor tazobactam, can solve the problems of danger and explosion, low total yield and the like, and achieves easy operation, mild reaction conditions, The effect of easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
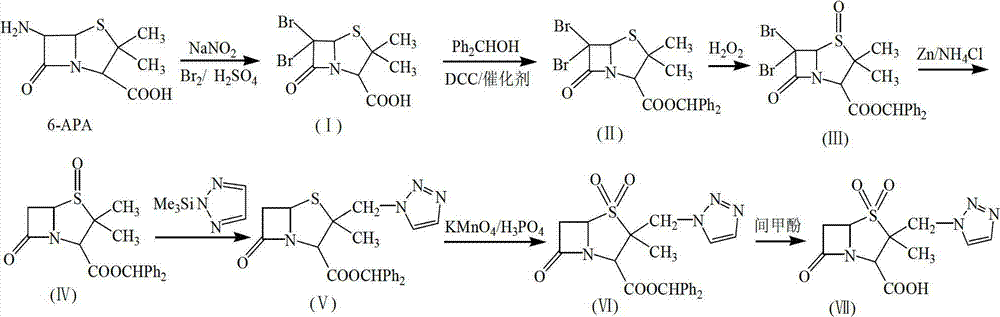
[0061] (1) Add CH to a 1000mL three-necked bottle 2 Cl 2 300mL and 1.5mol.L -1 h 2 SO 4 100mL, stirred and cooled to 0°C, then added 36.5g (0.228mol) of bromine, 0.33g of hexadecyltrimethylammonium bromide and 10.5g (0.152mol) of sodium nitrite into the reaction liquid, and continued stirring to dissolve Then add 33g (0.152mol) of 6-APA in batches, and after stirring for 1h at 0-5°C, add 1mol.L -1 NaHSO 3 Until the solution is tested with KI-starch test paper and does not change color. Then let the layers stand, and the water layer was washed with 100mL CH 2 Cl 2 After two extractions, the organic layers were combined, followed by water, 7% NaHCO 3 Aqueous solution, saturated sodium chloride aqueous solution washing, the obtained CH containing 6,6-dibromopenicillanic acid 2 Cl 2 The solution was directly used in the next reaction.
[0062] (2) Add CH of 6,6-dibromopenicillanic acid in a 1000mL three-necked bottle 2 Cl 2 Solution (about 400ml), after cooling down ...
Embodiment 2
[0070] (1) Add CH to a 1000mL three-necked bottle 2 Cl 2 300mL and 1.5mol.L -1 h 2 SO 4 100mL, stirred and cooled to 0°C, then added 73g (0.456mol) of bromine, 1.65g of hexadecyltriethylammonium bromide and 10.5g (0.152mol) of sodium nitrite into the reaction liquid, and kept stirring, after dissolving Add 33g (0.152mol) of 6-APA in batches, and after stirring for 1h at 0-5°C, add 1mol.L -1 NaHSO 3 Until the solution is tested with KI-starch test paper and does not change color. Then let the layers stand, and the water layer was washed with 100mLCH 2 Cl 2 After two extractions, the organic layers were combined, followed by water, 7% NaHCO 3 Aqueous solution, saturated sodium chloride aqueous solution washing, the obtained CH containing 6,6-dibromopenicillanic acid 2 Cl 2 The solution was directly used in the next reaction.
[0071] (2) Add CH of 6,6-dibromopenicillanic acid in a 1000mL three-necked bottle 2Cl 2 Solution (about 400ml), after cooling down to 5°C,...
Embodiment 3
[0078] (1) Add CH to a 1000mL three-necked bottle 2 Cl 2 300mL and 1.5mol.L -1 h 2 SO 4 100mL, stirred and cooled to 0°C, then added 122g (0.762mol) of bromine, 2.2g of dodecyltrimethylammonium bromide and 35g (0.508mol) of sodium nitrite into the reaction solution, and kept stirring, dissolved and divided Add 55g (0.254mol) of 6-APA in batches, and after stirring for 1h at 0-5°C, add 1mol.L -1 NaHSO 3 Until the solution is tested with KI-starch test paper and does not change color. Then let the layers stand, and the water layer was washed with 100mL CH 2 Cl 2 After two extractions, the organic layers were combined, followed by water, 7% NaHCO 3 Aqueous solution, saturated sodium chloride aqueous solution washing, the obtained CH containing 6,6-dibromopenicillanic acid 2 Cl 2 The solution was directly used in the next reaction.
[0079] (2) Add CH of 6,6-dibromopenicillanic acid in a 1000mL three-necked bottle 2 Cl 2 Solution (about 400ml), after cooling down to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com