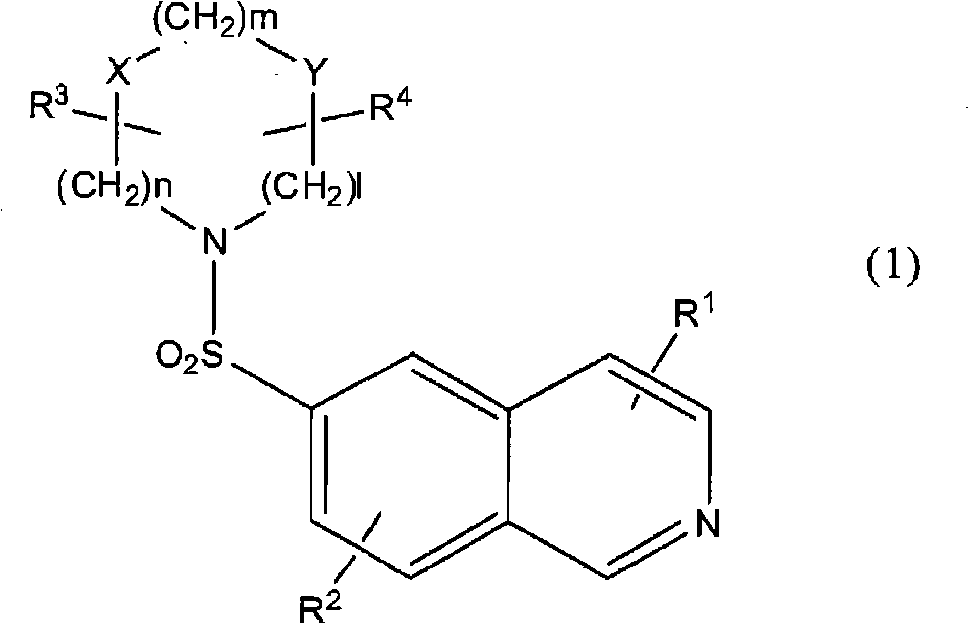
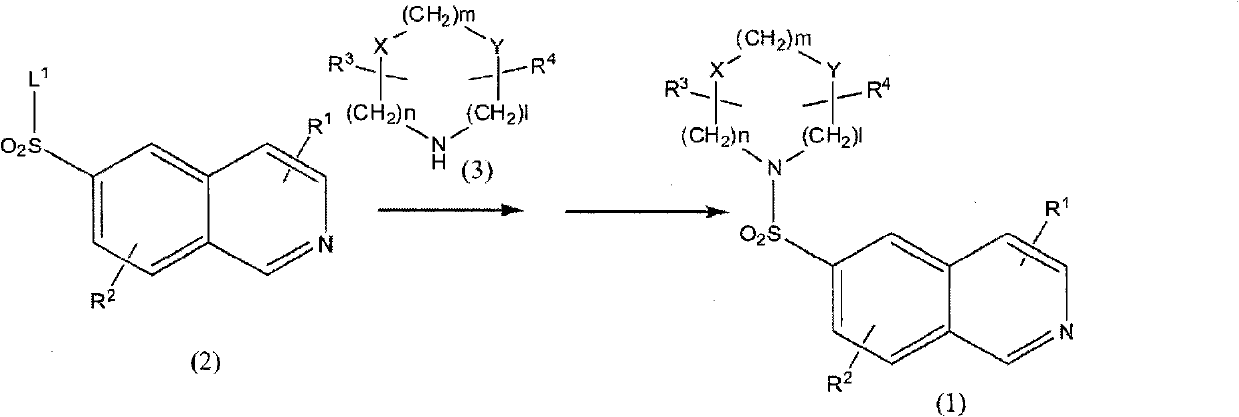
Substituted isoquinoline derivative
A technology of isoquinoline and derivatives, applied in the field of isoquinoline-6-sulfonamide derivatives, to achieve excellent effect of lowering intraocular pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0172] Production examples of synthetic intermediates and compounds of the present invention, and evaluation examples of biological activity are shown below. It should be noted that these illustrations are for better understanding of the present invention and do not limit the scope of the present invention. In addition, unless otherwise specified, Boc in the chemical structural formula represents a tert-butoxycarbonyl group.
reference example 1
[0175] Synthesis of 6-aminoisoquinoline (reference compound 1)
[0176]
[0177]17.2 g of 6-bromoisoquinoline (refer to WO 2008 / 077553) was weighed in an autoclave, 200 mL of 28% ammonia water and 10.8 g of copper sulfate pentahydrate were added and sealed, and stirred at 190 degrees for 6 hours. After cooling to room temperature, the reaction solution was poured into 250 mL of 10% aqueous sodium hydroxide solution, and extracted with ethyl acetate (100 mL×5). The extract was dried over anhydrous sodium sulfate, filtered, and then concentrated. The obtained crude product was suspended with dichloromethane, and filtered off to obtain 10.2 g (85%) of the target product as light brown crystals.
[0178] 1 H-NMR spectrum (CDCl 3 , δppm): 5.54(br s, 2H), 6.58(s, 1H), 7.00(d, J=9.0Hz, 1H), 7.35(d, J=5.5Hz, 1H), 7.75(d, J=9.0 Hz, 1H), 8.32(d, J=5.5Hz, 1H), 8.98(s, 1H)
reference example 2
[0180] Synthesis of 6-chlorosulfonylisoquinoline (reference compound 2)
[0181]
[0182] Suspend 4.0 g of 6-aminoisoquinoline (reference compound 1) in 40 mL of concentrated hydrochloric acid (35%) at 0°C, add 4.0 g of sodium nitrite little by little, and stir for 30 minutes. This reaction solution was dropped into a mixed solution of 20 mL of acetic acid saturated with sulfur dioxide gas generated from sodium bisulfite and sulfuric acid and 298 mg of copper chloride at 0° C., and stirred for 1 hour. It was neutralized by adding saturated aqueous sodium bicarbonate solution, and extracted with dichloromethane (100 mL×2). The organic layer was washed with saturated brine and dried over anhydrous sodium sulfate. Since the target product was unstable, the obtained dichloromethane solution was used in the following reaction without further purification.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com