Method for preparing catalyst by hydrotreatment
A hydrotreating and catalyst technology, applied in physical/chemical process catalysts, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc. It can improve the activity, reduce the rate of activity loss, and enhance the promotion effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
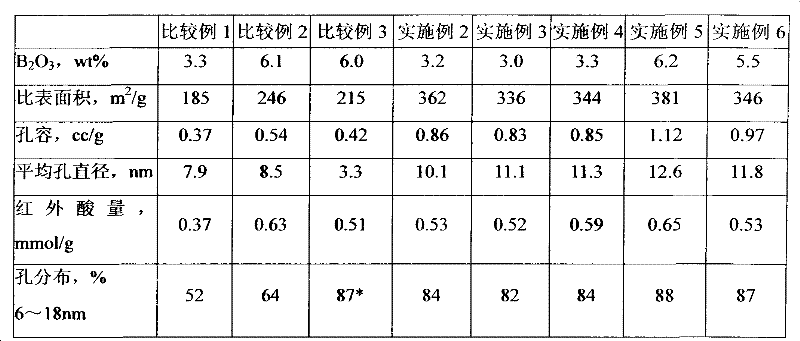
[0031] will contain Al 2 o 3 5g / 100mL AlCl 3 Solution 2000mL with NH 3 10g / 100mL of ammonia water was added dropwise into the gelling reaction tank stirred at a temperature of 60°C, and the pH value was maintained at 7.6, and the reaction contact time was 40min. Aging was performed at a temperature of 70°C and a pH of 8.0. The aging time is 1h. The product was filtered, and then washed with deionized water with a solid-to-liquid volume ratio of 1:20 at a washing temperature of 80° C. for three times. The obtained filter cake was dried at 120° C. for 3 h to obtain aluminum hydroxide.
[0032] Take 100g of the above-mentioned aluminum hydroxide, mix it with 110g of SB alumina powder peptized by nitric acid, knead for 20min, make a plastic paste, and extrude it through a clover orifice plate with a diameter of 1.4mm. After the wet strip was dried at 130°C for 8h, it was calcined at 520°C for 4h to obtain alumina carrier S-1.
Embodiment 2
[0034] After the gelling reaction of Example 1, add the 2 o 3 3g / 100mL organic compound solution 100mL. The boron-containing organic compound solution is prepared by dissolving boric acid in water, and then the molar ratio of boric acid to mannitol is 1:1. The preparation of other aluminum hydroxide is the same as in Example 1 to obtain boron-containing aluminum hydroxide.
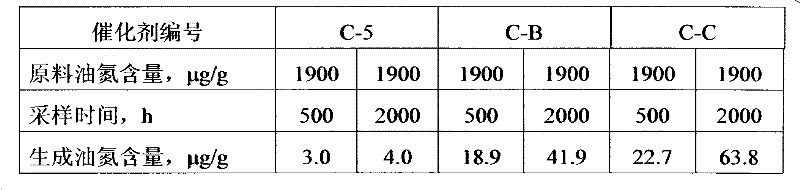
[0035] Take 120g of the above-mentioned boron-containing aluminum hydroxide, mix it with 126g of SB alumina powder peptized by nitric acid and an aqueous solution containing molybdenum and cobalt, and knead for 20 minutes to make a plastic paste, and extrude it through a clover orifice plate of φ1.4mm. The wet bar was dried at 130°C for 3 hours, then calcined at a constant temperature of 220°C for 2 hours, at a constant temperature of 320°C for 2.0 hours, and at a constant temperature of 530°C for 3.0 hours to obtain a catalyst C-2 intermediate, in which MoO 3 The weight content of CoO is 11.1%, and the ...
Embodiment 3
[0037] The boron-containing organic compound solution in Example 2 was replaced by the boric acid to ethylene glycol molar ratio of 1.0:1.5, and other aluminum hydroxide was prepared in the same way as in Example 2 to obtain boron-containing aluminum hydroxide.
[0038] Take 110g of the above-mentioned boron-containing aluminum hydroxide, mix it with 118g of SB alumina powder peptized by nitric acid and an aqueous solution containing molybdenum and phosphorus, knead for 20 minutes, and make a plastic paste, and extrude it through a clover orifice plate with a diameter of 1.4mm. The wet bar was dried at 140°C for 3h, then calcined at a constant temperature of 200°C for 0.5h, at a constant temperature of 320°C for 2.0h, and at a constant temperature of 530°C for 3.0h to obtain the catalyst C-3 intermediate, in which MoO 3 The weight content of P is 23.2%, and the weight content of P is 0.85%. The catalyst C-3 intermediate was impregnated with the remaining molybdenum, nickel and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com