Posaconazole orally taken suspension and preparation method thereof
A posaconazole mouth, posaconazole technology, applied in the field of medicine, can solve the problems of product content fluctuation range, long process time, large main drug loss, etc., to achieve stable and qualified product content, simple process steps, and high quality Guaranteed effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
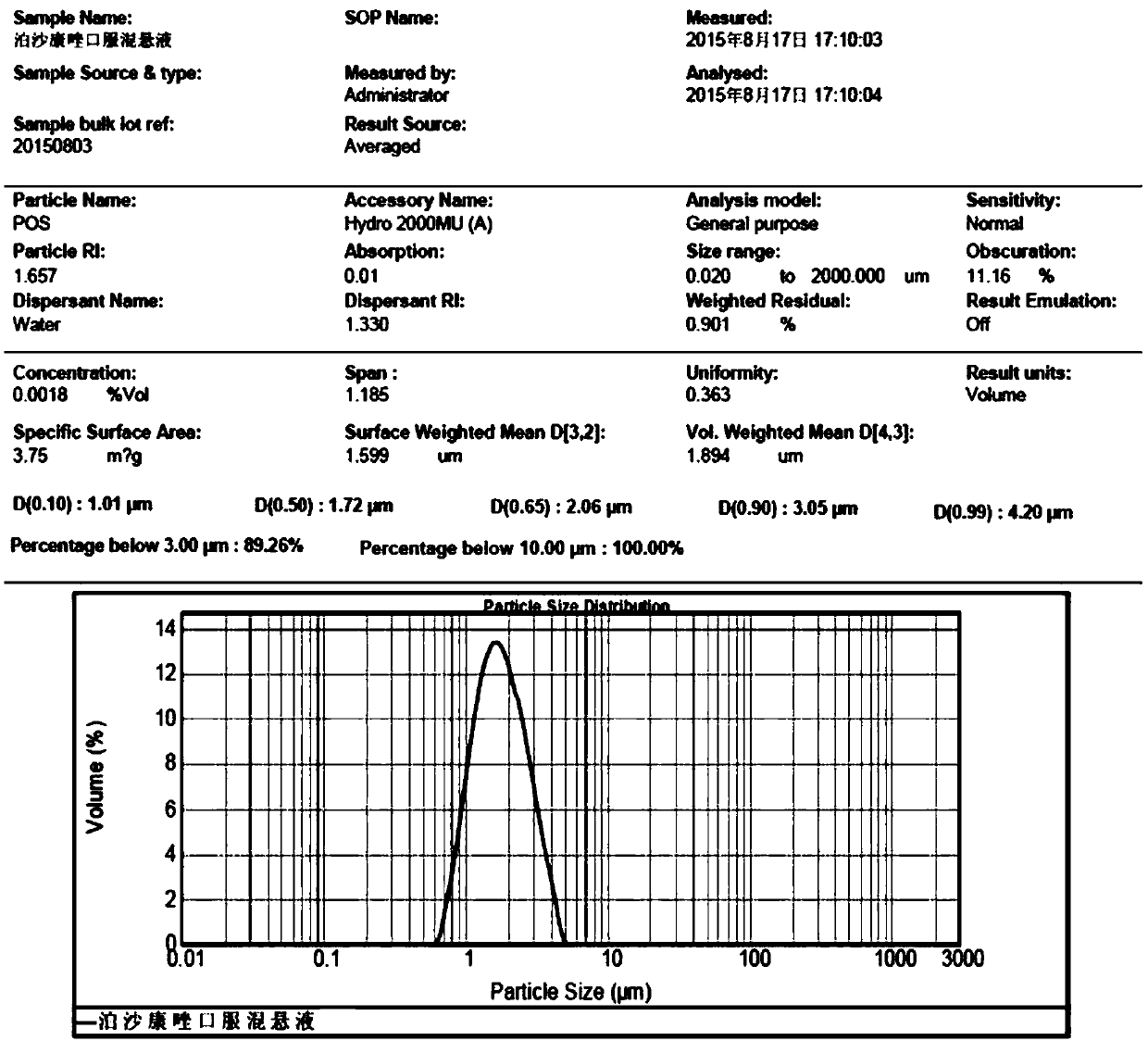
[0052] Example 1 is used to prepare the composition of posaconazole oral suspension by microcrystallization process
[0053] The composition for preparing posaconazole oral suspension by microcrystallization process is composed of the following components in parts by weight: posaconazole 35mg, polysorbate 80 8mg, simethicone 3mg, sodium benzoate 2mg , sodium citrate 0.6mg, citric acid 1.5mg, xanthan gum 3mg, fructose syrup 160mg, titanium dioxide 3mg, essence 4mg, glycerin 320mL and water medium.
Embodiment 2
[0054] Example 2 is used for the microcrystallization process to prepare the composition of posaconazole oral suspension
[0055] The composition used to prepare posaconazole oral suspension by microcrystallization process consists of the following components in parts by weight: posaconazole 45mg, polysorbate 80 10mg, simethicone 4mg, sodium benzoate 2mg , sodium citrate 0.6mg, citric acid 1.5mg, xanthan gum 4mg, fructose syrup 150mg, titanium dioxide 4mg, essence 3mg, glycerin 300mL and water medium.
Embodiment 3
[0056] Example 3 Composition for preparing posaconazole oral suspension by microcrystallization process
[0057] The composition for the preparation of posaconazole oral suspension by microcrystallization process consists of the following components in mg by weight: posaconazole 40mg, polysorbate 80 8mg, simethicone 3mg, sodium benzoate 2mg , sodium citrate 0.6mg, citric acid 1.5mg, xanthan gum 3mg, fructose syrup 340mg, titanium dioxide 4mg, essence 5mg, volume percentage is 200mL of 95% ethanol solution and water medium. Ethanol is filtered out during the process and is not carried into the finished product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com