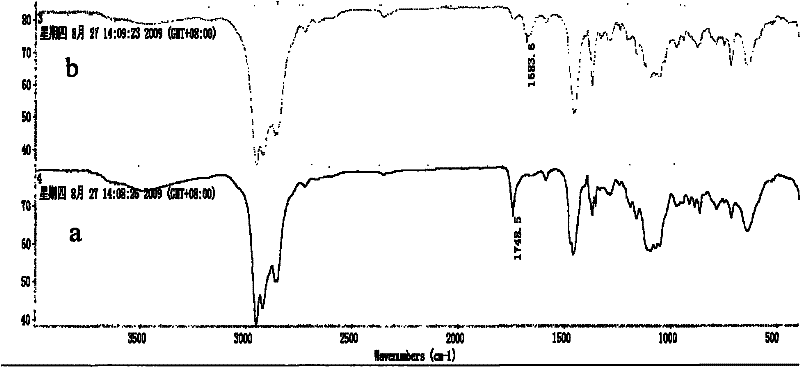
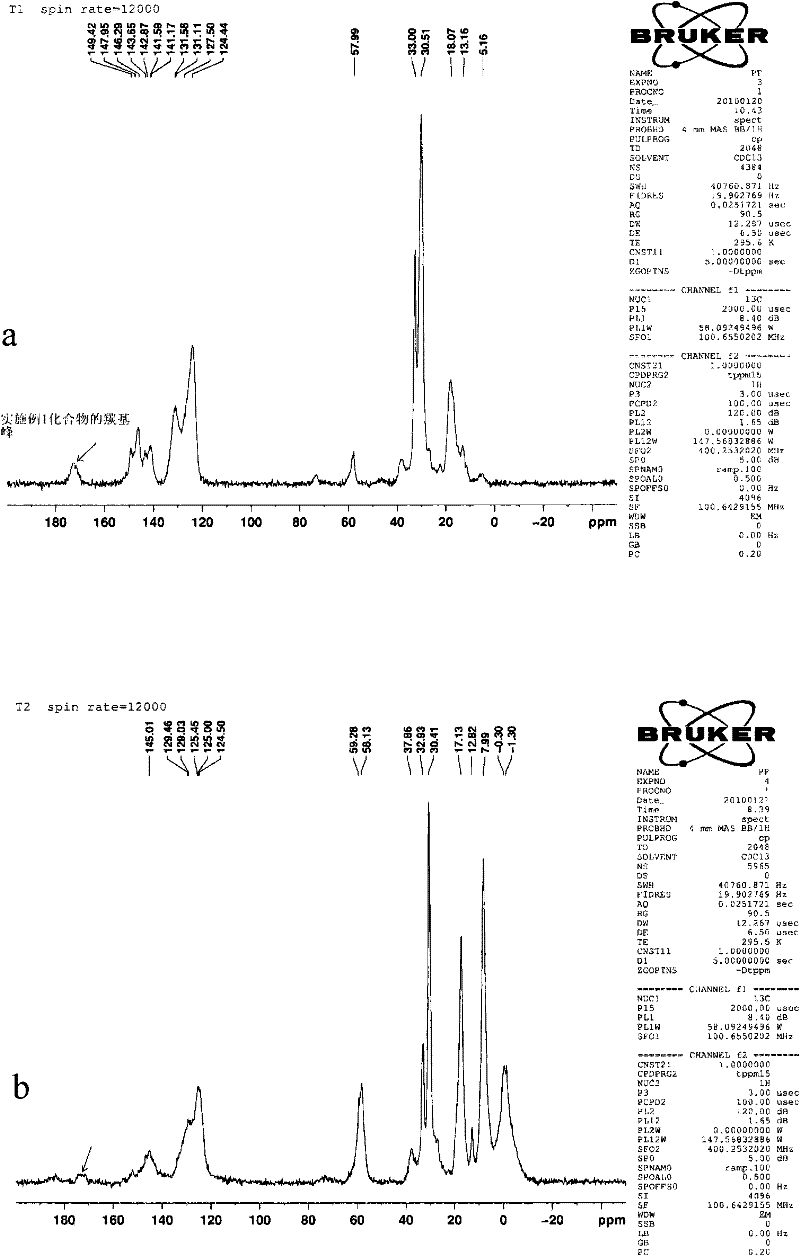
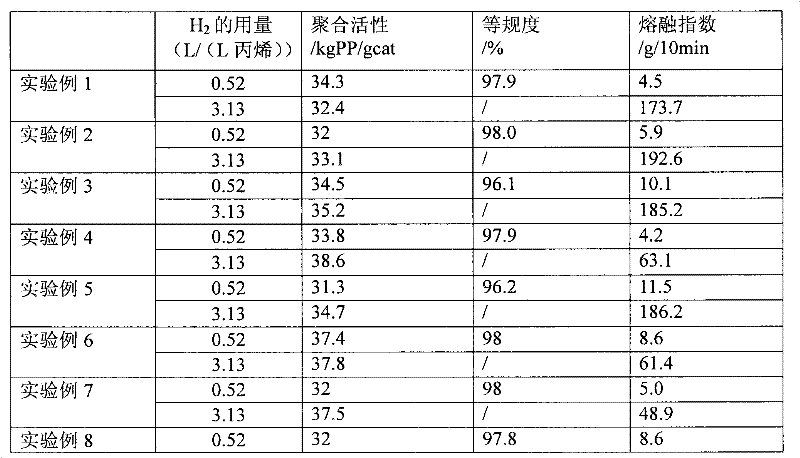
External donor compound
An external electron donor and compound technology, which is applied in the field of external electron donor compounds to achieve high stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0098] According to the present invention, the compound represented by formula 3 can be synthesized by conventional methods in the field of organic synthesis, without special limitation. Preferably, in the present invention, the preparation method of the calixarene compound shown in formula 3 comprises: under etherification conditions or esterification conditions, the calixarene shown in formula 6 and R 5 x 3 Contact is carried out at a molar ratio of 1:3-3.6, and the resulting product is then mixed with R 7 wxya 5 (i.e., Grignard reagent) or R 8 Li (ie, organolithium compound) is mixed at a molar ratio of 1:1-1.2 at a temperature of -70°C to 10°C, preferably at a temperature of -30°C to 10°C, and the resulting mixture is heated at 40-80°C, preferably The reaction is carried out at a temperature of 40-60°C, wherein, R 7 and R 8 Each is C 1 -C 5straight or branched chain alkyl, X 3 and x 5 Each is a halogen, such as one of chlorine, bromine and iodine, preferably chlor...
Embodiment 1
[0231] Example 1: 4-tert-butylcalix[4]arene-O, O', O"-triisobutyryl-O"'-(di-n-butylaminodiethoxy) silane (that is, in formula I, R 1 Both are ethyl, R 3 Both are n-butyl, in formula II, R 5 Both are isobutyryl, R 6 are all tert-butyl) synthesis:
[0232] (1) Preparation of 4-tert-butylcalix[4]arene
[0233] With reference to the method disclosed in p-tert-butylcalix [4] arene (p-tert-Butylcalix [4] arene) (Org.Synth, 68,324, 1990), prepare 4-tert-butyl calix [4] according to the following steps ]Aromatics:
[0234] Under nitrogen protection, in a 2500mL three-neck flask equipped with a stirring device, dropping funnel and water separator, add 100g p-tert-butylphenol, 70mL concentration is 37% by weight of formaldehyde and 1g sodium hydroxide (dissolved in 5g water) . Under the protection of nitrogen, after stirring evenly, place the three-necked flask in an oil bath, heat it to reflux, and separate the water from the water separator. After no more water is separated from ...
Embodiment 2
[0245] Example 2: 4-tert-butylcalix[4]arene-O, O', O"-triisobutyryl-O"'-(diisobutylaminodiethoxy) silane (that is, in formula I, R 1 Both are ethyl, R 3 are isobutyl, in formula II, R 5 Both are isobutyryl, R 6 are all tert-butyl) synthesis:
[0246] The title compound was synthesized by the same method as in Example 1, except that diisobutylamine was used instead of di-n-butylamine. h 1 NMR (300MHz, CDCl 3 , δ): 7.06 (6H, phenyl), 6.71 (2H, Ph), 3.83 (4H, CH 2 ), 3.80 (8H, CH 2 ), 2.68 (3H, CH), 2.52 (4H, CH 2 ), 1.67 (2H, CH), 1.35 (36H, CH 3 ), 1.20 (6H, CH 3 ), 1.09 (18H, CH 3 ), 0.9 (12H, CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com