Polysaccharide conjugate vaccine and preparation method thereof
A technology combining vaccines and polysaccharides, applied in the field of vaccines, can solve problems such as bacterial polysaccharide conjugates that have not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
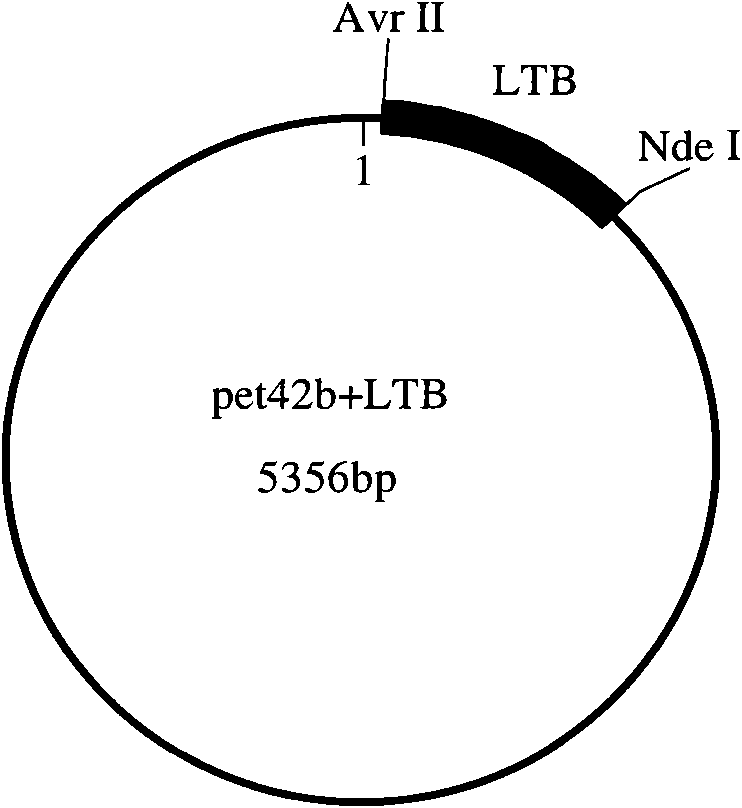
[0036] The present invention will be described in detail below by taking the combination of Escherichia coli heat-labile enterotoxin B subunit (LTB) and group C meningococcal polysaccharide (GCMP) as an example.
[0037] (1) Cloning and expression of recombinant Escherichia coli heat-labile enterotoxin B subunit (rLTB) 1) Synthesis of LTB gene and construction of expression vector
[0038] The LTB gene sequence is selected from GENBANK, and the sequence number is GI: 157021177. According to the Escherichia coli codon usage frequency table (references: Maloy, S., V.Stewart, and R.Taylor.1996.Genetic analysis of pathogenic bacteria.Cold SpringHarbor Laboratory Press, NY.) Optimize this sequence, add Add 6 histidine tags and stop codons, and then add NdeI and AvrII restriction sites at both ends of the sequence.
[0039] Artificially synthesize the above-mentioned LTB gene, then use NdeI and AvrII to double-enzyme digest LTB and pET42b (purchased from Novagen), and then connect ...
Embodiment 2
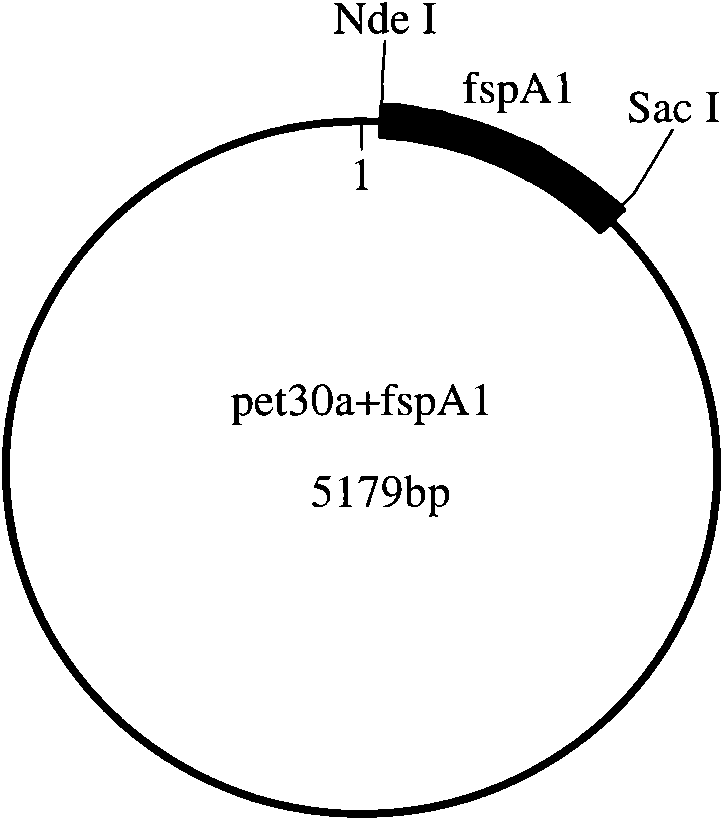
[0090] The present invention will be described in detail below by taking the combination of Campylobacter jejuni flagellar secretory protein A1 (FspA1) and group A meningococcus polysaccharide (GAMP) as an example.
[0091] (1), Cloning and expression of FspA1
[0092] 1) Synthesis of FspA1 gene and construction of expression vector
[0093] The FspA1 gene sequence is selected from GENBANK, and the sequence number is GI: 116292639. According to the Escherichia coli codon usage frequency table (references: Maloy, S., V.Stewart, and R.Taylor.1996.Genetic analysis of pathogenic bacteria.Cold SpringHarbor Laboratory Press, NY.) Optimize this sequence, add Add 6 histidine tags and stop codons, and then add NdeI and SacI restriction sites at both ends of the sequence respectively.
[0094] The above-mentioned FspA1 gene was artificially synthesized, and then FspA1 and pET30a (purchased from Novagen) were double-digested with NdeI and SacI, and then the target fragment after double...
Embodiment 3
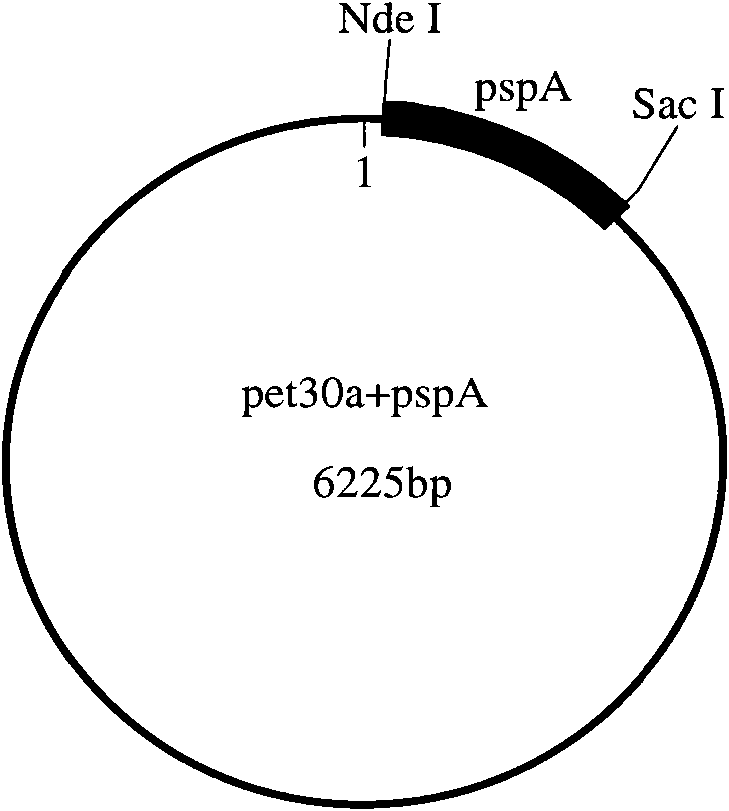
[0122] The present invention will be described in detail below by taking the combination of pneumococcal surface membrane protein A (PspA) and group A meningococcal polysaccharide (GAMP) as an example.
[0123] (1), cloning and expression of recombinant PspA
[0124] 1) Synthesis of PspA gene and construction of expression vector
[0125] The PspA gene sequence is selected from GENBANK, and the sequence number is GI:193804931. According to the Escherichia coli codon usage frequency table (references: Maloy, S., V.Stewart, and R.Taylor.1996.Genetic analysis of pathogenic bacteria.Cold SpringHarbor Laboratory Press, NY.) Optimize this sequence, add Add 6 histidine tags and stop codons, and then add NdeI and SacI restriction sites at both ends of the sequence respectively.
[0126] The above-mentioned PspA gene was artificially synthesized, and then PspA and pET30a (purchased from Novagen) were double-digested with NdeI and SacI, and then the target fragment after double-digest...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com