Method for producing isopropylbenzene
A kind of technology of cumene and dicumene, applied in the field of producing cumene, can solve the problem of high n-propylbenzene content, and achieve the effects of reducing content, good technical effect and improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
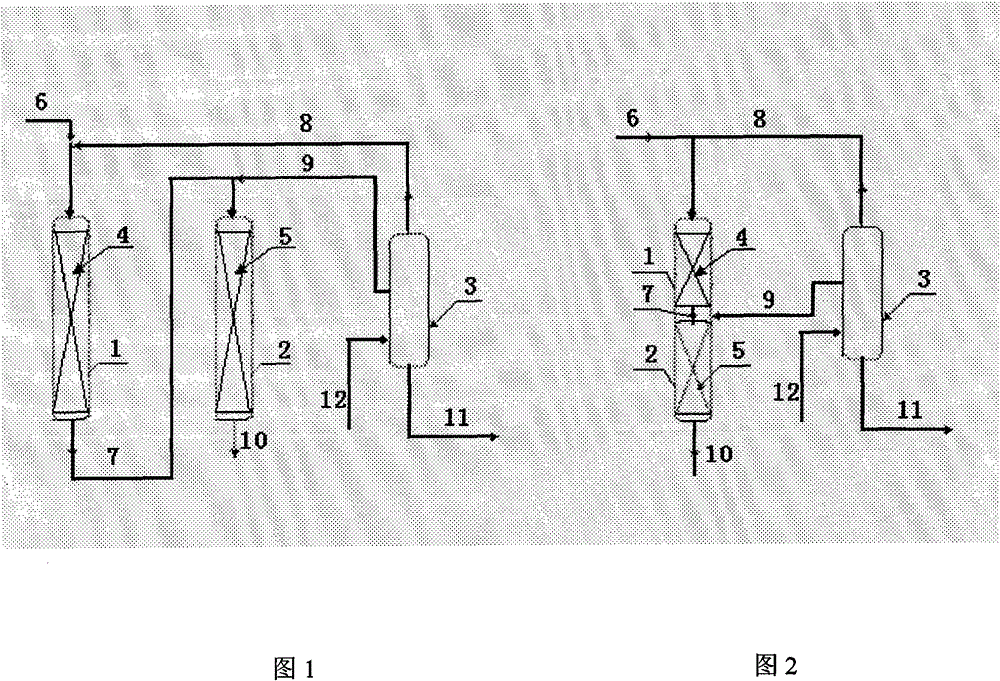
[0026] according to figure 1 The process flow, the first transalkylation reaction zone and the second transalkylation reaction zone are independent, two fixed-bed reactors connected in series. The first transalkylation reaction zone is loaded with 15 grams of shaped catalyst of Beta zeolite, and the second transalkylation reaction zone is loaded with 60 grams of shaped catalyst of SHY-1 zeolite. The reaction conditions in the first transalkylation reaction zone are: 150°C of reaction temperature, 110 g / hour of benzene flow (stream 6), 20 g / hour of multi-substituted cumene (stream 8), and diisopropyl benzene in stream 8 The benzene content is 99%. The reaction conditions of the second transalkylation reaction zone are as follows: reaction temperature 178° C., outlet pressure of the second reaction zone 1.5 MPa, stream 9 flow rate 90 g / hour, triisopropylbenzene content in stream 9 6%. Continuous reaction for 5 days.
[0027] The operating conditions of the multi-substituted c...
Embodiment 2
[0030] according to figure 1 The process flow, the first transalkylation reaction zone and the second transalkylation reaction zone are independent, two fixed-bed reactors connected in series. The first transalkylation reaction zone is loaded with 50 grams of Beta zeolite shaped catalyst, and the second transalkylation reaction zone is loaded with 30 grams of SHY-2 zeolite shaped catalyst. The reaction conditions in the first transalkylation reaction zone are: 148°C of reaction temperature, 100 g / hour of benzene flow (stream 6), 50 g / hour of multi-substituted cumene (stream 8), and diisopropyl benzene in stream 8 The benzene content is 99%. The reaction conditions in the second transalkylation reaction zone are: 185°C of reaction temperature, 1.5MPa of the second reaction zone outlet pressure, 25 grams / hour of multi-substituted cumene (streaming 9) flow rate, and 8% triisopropylbenzene content in the stream 9 . Continuous reaction for 5 days.
[0031] The operating conditi...
Embodiment 3
[0034] according to figure 1 The process flow, the first transalkylation reaction zone and the second transalkylation reaction zone are independent, two fixed-bed reactors connected in series. The first transalkylation reaction zone was loaded with 40 grams of shaped catalyst of Beta zeolite, and the second transalkylation reaction zone was loaded with 40 grams of shaped catalyst of MCM-49 zeolite. The reaction conditions in the first transalkylation reaction zone are: 151°C of reaction temperature, 80 grams / hour of benzene flow (stream 6), 40 grams / hour of multi-substituted cumene (stream 8), and diisopropyl in stream 8. The benzene content is 98%. The reaction conditions in the second transalkylation reaction zone are: 171°C of reaction temperature, 1.5MPa of the second reaction zone outlet pressure, 50 grams / hour of multi-substituted cumene (streaming 9) flow rate, and 5% triisopropylbenzene content in the stream 9 . Continuous reaction for 5 days.
[0035] The operatin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com