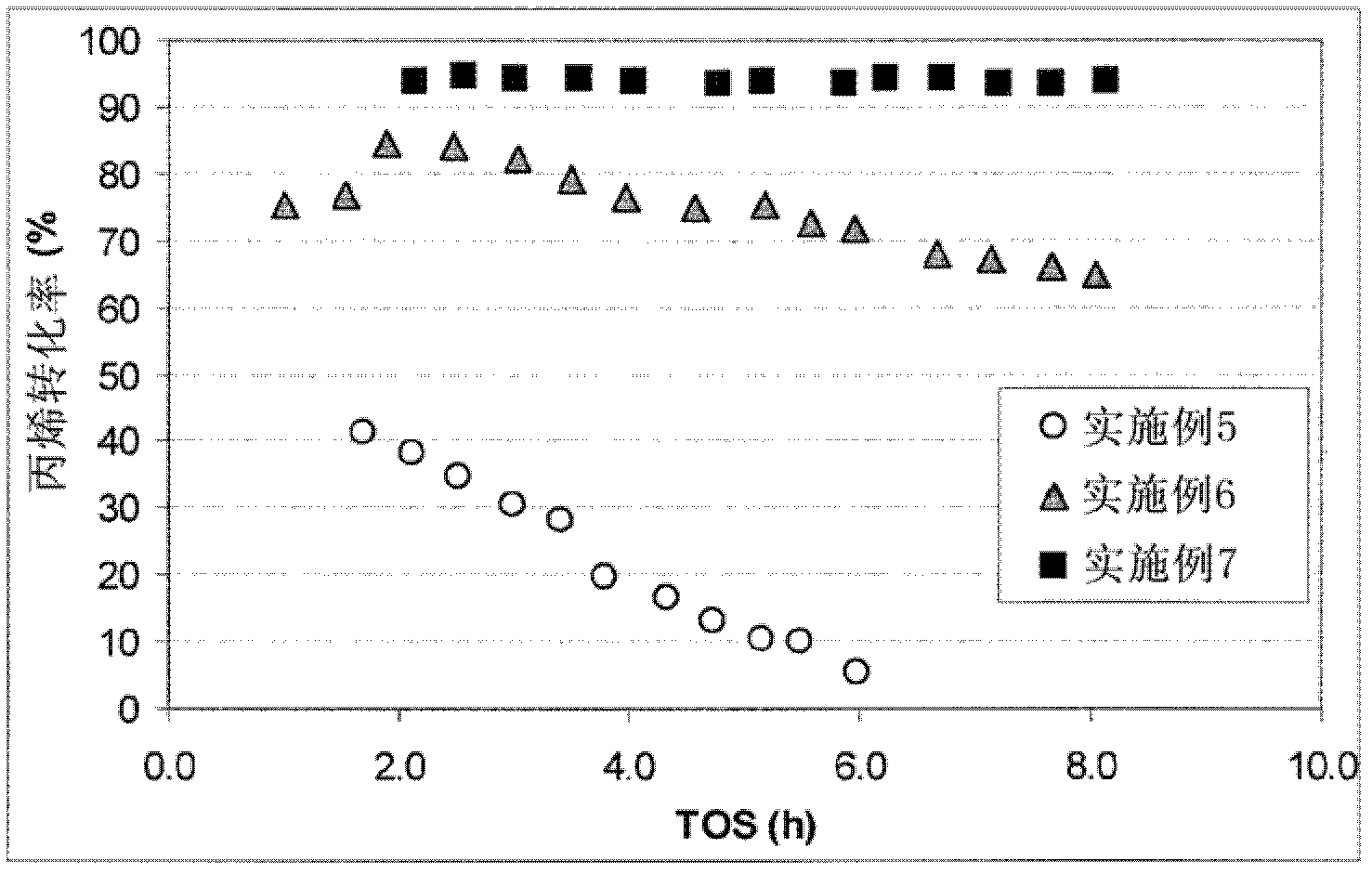
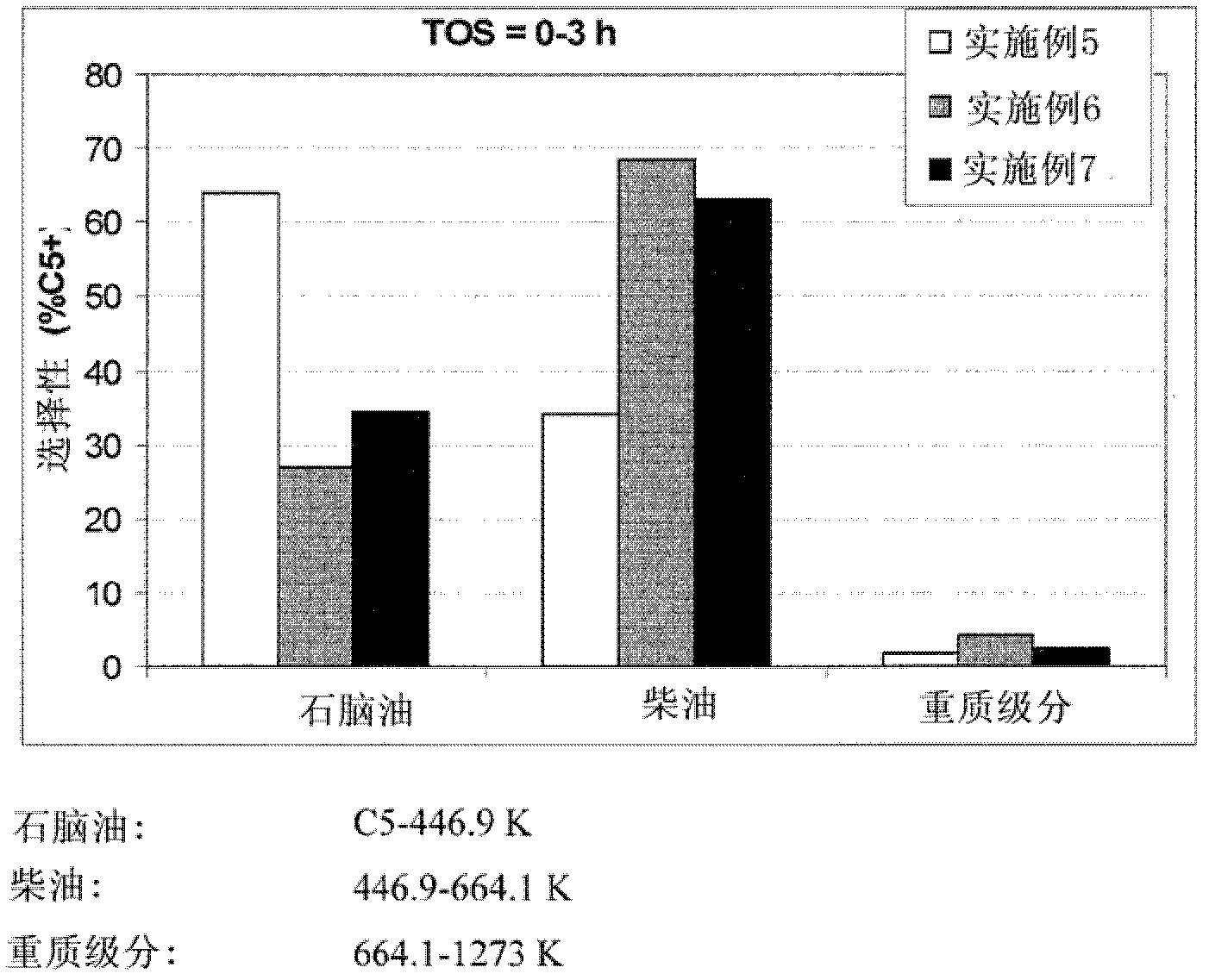
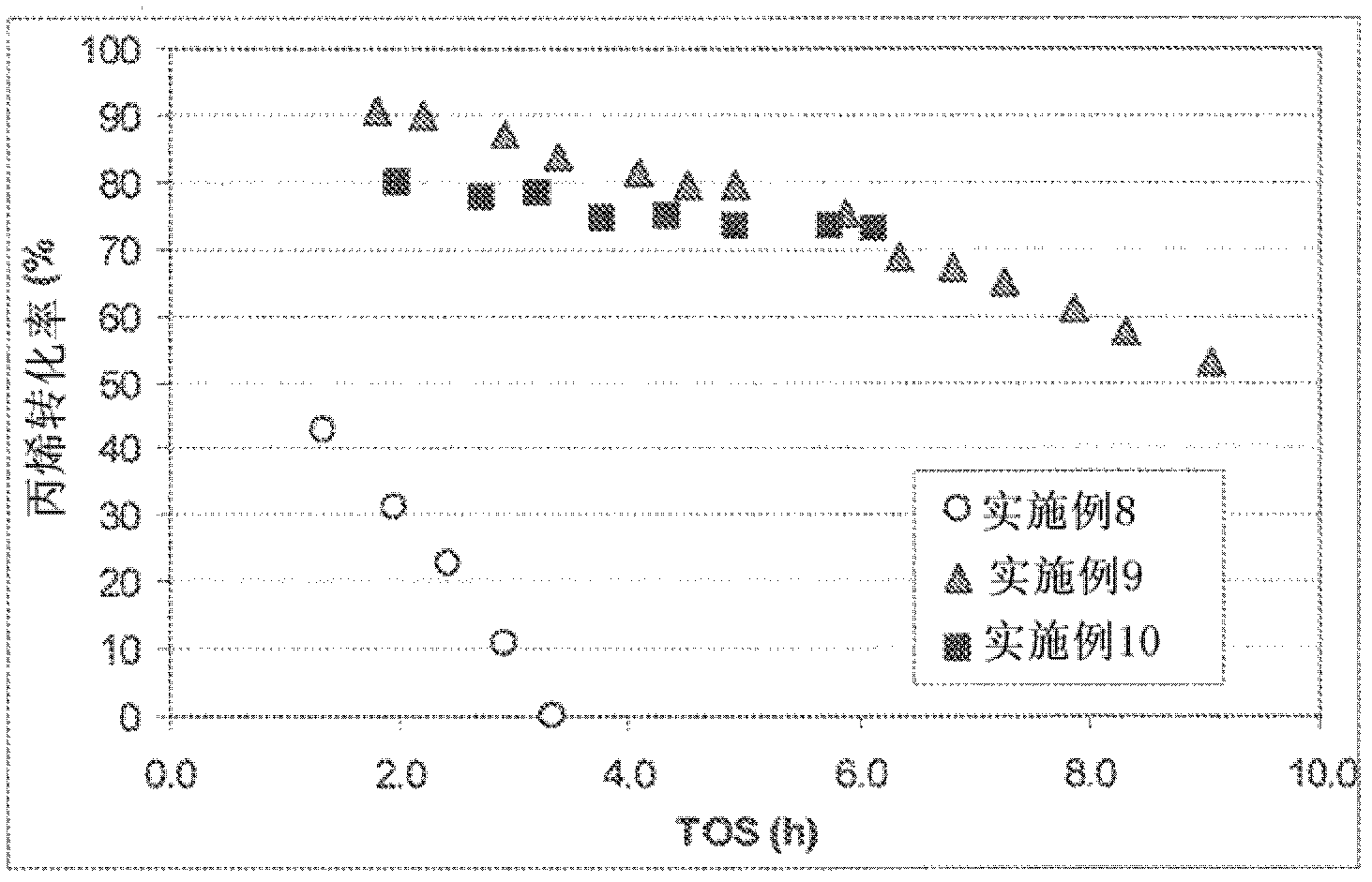
Modified zeolite catalyst
A technology for modifying zeolite and catalyst, applied in catalysts, molecular sieve catalysts, carbon compound catalysts, etc., can solve problems such as deactivation and blocking, and achieve the effect of improving selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 3 g of zeolite θ1 in the hydrogen form (H-θ-1, Si / Al=50) was suspended in 100 ml of 0.2 M aqueous sodium hydroxide solution and vigorously stirred at 80° C. for 30 minutes. The reaction was then quenched by cooling in an ice bath. The remaining solid was isolated by filtration, washed with distilled water and dried overnight at 100°C. Then use 0.1M NH4NO 3 Three consecutive exchanges of the solution at 83 °C for 2 h and using a solution-to-solid weight ratio of 20 converted base-treated θ-1 to its acidic form. Finally the samples were calcined at 450 °C for 5 hours. When studied using transmission electron microscopy and nitrogen absorption (77°K) and the BJH method (V 中孔 =0.107cm 3 g -1 ) measured, the product of this method shows distinct mesopores compared to untreated zeolites.
Embodiment 2
[0042] 3 g of zeolite θ-1 in the hydrogen form (H-θ-1, Si / Al=50) was suspended in 100 ml of 0.2 M aqueous sodium hydroxide solution and vigorously stirred at 85° C. for 30 minutes. The reaction was then quenched by cooling in an ice bath. The remaining solid was isolated by filtration, washed with distilled water and dried overnight at 100°C. The base-treated θ-1 was then suspended in 2.0M aqueous oxalic acid solution (solution / solid ratio 10 wt / wt) and stirred at 70°C for 2 hours. The solid was isolated by filtration, washed with distilled water and dried overnight at 100°C. Finally the samples were calcined at 375°C for 3 hours. When studied using transmission electron microscopy and nitrogen absorption (77°K) and the BJH method (V 中孔 =0.117cm 3 g -1 ) measured, the product of this method shows distinct mesopores compared to untreated zeolites.
Embodiment 3
[0043] Embodiment 3 (comparative example)
[0044] 3 g of the theta-1 zeolite in the hydrogen form (H-θ-1, Si / Al=25) used in Example 8 was suspended in 100 ml of 0.2 M aqueous sodium hydroxide solution and vigorously stirred at 85° C. for 30 minutes . The reaction was then quenched by cooling in an ice bath. The remaining solid was isolated by filtration, washed with distilled water and dried overnight at 100°C. Then use 0.1M NH4NO 3 Three consecutive exchanges of the solution at 80 °C for 2 h and using a solution-to-solid weight ratio of 20 converted base-treated θ-1 to its acidic form. Finally the samples were calcined at 450 °C for 5 hours. When studied using transmission electron microscopy and nitrogen absorption (77°K) and the BJH method (V 中孔 =0.067cm 3 g -1 ) measured, the product of this method showed fewer mesopores compared to untreated zeolites. Although not in accordance with the present invention, this example demonstrates that mesopores can be introduced...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com