Method for quantification of total phosphorous
A phosphorus compound and time-specified technology, applied in the quantitative field of total phosphorus, can solve the problems of quantitative result fluctuation and lack of reliability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0083] unit
[0084] The unit of mg[P] / L represents the number of micrograms of phosphorus contained in 1L of water.
[0085] Reagents and Spectrophotometers
[0086] Reagents and spectrophotometers used in the following Examples and the like are as follows.
[0087] Phosphorus standard solution (for water quality testing): Wako Pure Chemical Industries, Ltd. No. 160-19241
[0088] Silicon standard solution: Wako Pure Chemical Industries, Ltd. No. 192-06031
[0089] 1M sulfuric acid (for capacity analysis): Wako Pure Chemical Industries Ltd. No. 198-09595
[0090] Hydrochloric acid (special grade reagent): Wako Pure Chemical Industries Co., Ltd. No. 080-01066
[0091] Potassium peroxodisulfate (for the determination of nitrogen and phosphorus): Wako Pure Chemical Industries, Ltd. No. 169-1189
[0092] Ammonium peroxodisulfate (special grade reagent): Wako Pure Chemical Industries Co., Ltd. No. 012-03285
[0093] Hexaammonium heptamolybdate tetrahydrate (special grade...
experiment example 1
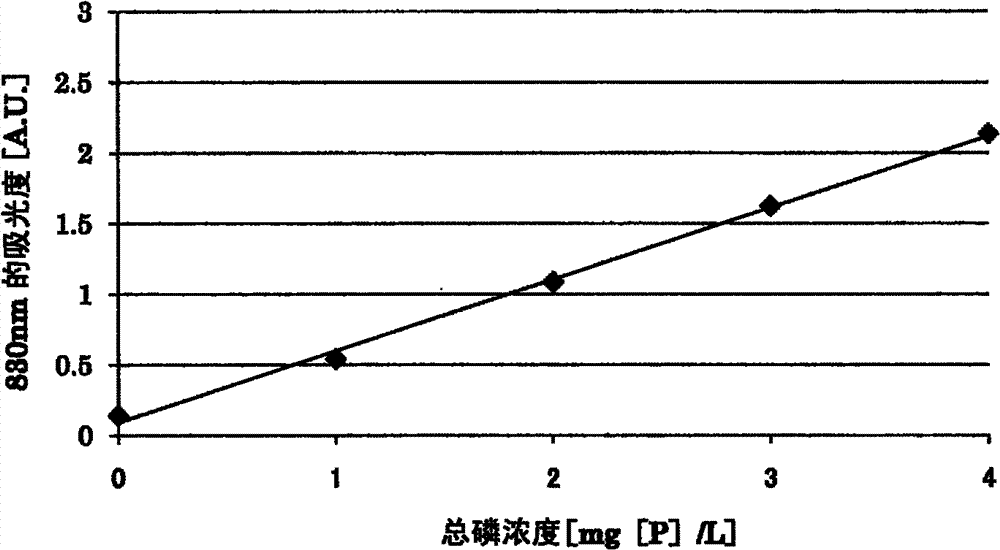
[0132] The following sample water and oxidizing agent aqueous solution were prepared. The D-glucose used in the preparation of the sample waters 2, 3, and 4 was assumed to be an organic substance as a mixture.
[0133]
[0134] Sample water 1:
[0135] Phosphate ion solution with a phosphate ion concentration of 5 mg[P] / L.
[0136] Sample water 2:
[0137] An aqueous solution obtained by dissolving sodium diphosphate decahydrate and D-glucose in distilled water (sodium diphosphate conversion concentration: 5 mg[P] / L, D-glucose concentration: 40 mg / L).
[0138] Sample water 3:
[0139] An aqueous solution obtained by dissolving adenosine-5'-triphosphate disodium trihydrate and D-glucose in distilled water (concentration in terms of adenosine-5'-triphosphate disodium is 5 mg[P] / L, D-glucose concentration is 40mg / L).
[0140] Sample water 4:
[0141] An aqueous solution obtained by dissolving disodium phenyl phosphate dihydrate and D-glucose in distilled water (concentrat...
experiment example 2
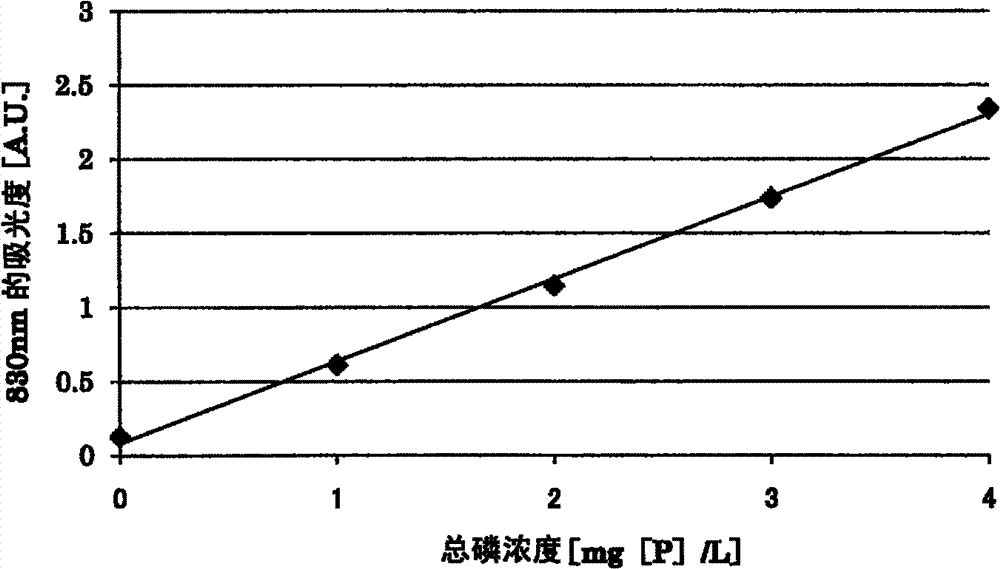
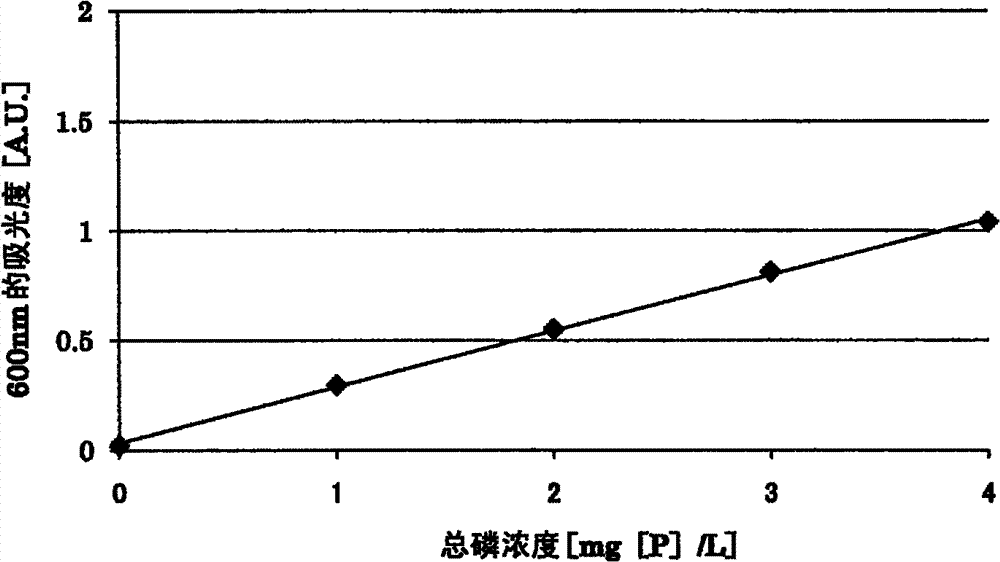
[0160] Prepare the following sample water. The D-glucose used in the preparation of the sample waters 5 and 6 is assumed to be an organic substance which is a mixture.
[0161] Sample water 5:
[0162] Adenosine-5'-triphosphate disodium trihydrate was prepared by dissolving adenosine-5'-triphosphate disodium trihydrate, phenyl phosphate disodium dihydrate and D-glucose in distilled water to a concentration of 1 mg [P ] / L, an aqueous solution with a concentration of disodium phenyl phosphate of 1 mg[P] / L and a concentration of D-glucose of 50 mg / L.
[0163] Sample water 6:
[0164] An aqueous solution having a sodium diphosphate concentration of 2 mg[P] / L and a D-glucose concentration of 50 mg / L was prepared by dissolving sodium diphosphate decahydrate and D-glucose in distilled water.
[0165] To 5 mL of each sample water, 0.8 mL of an aqueous potassium peroxodisulfate solution having a concentration of 30 g / L and 1.2 mL of 1M sulfuric acid were added. Then, each sample wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com