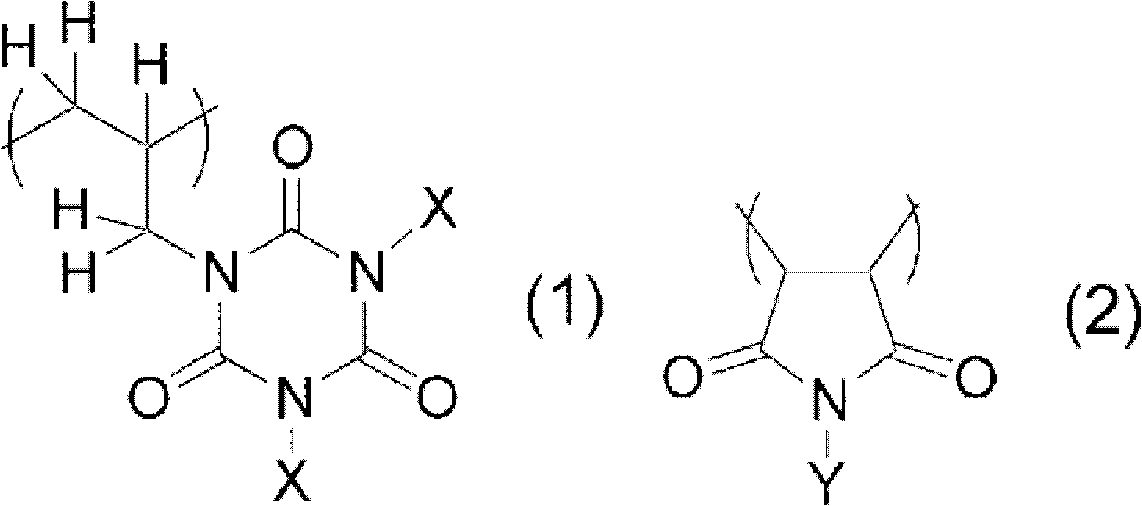
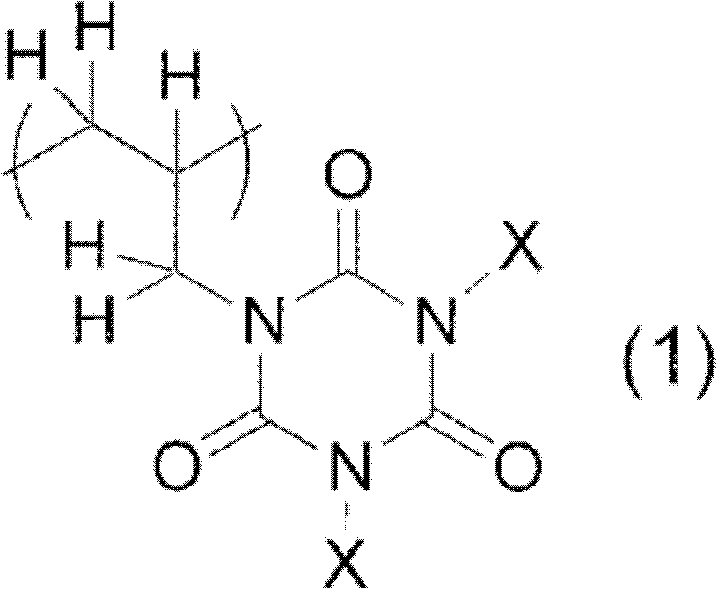
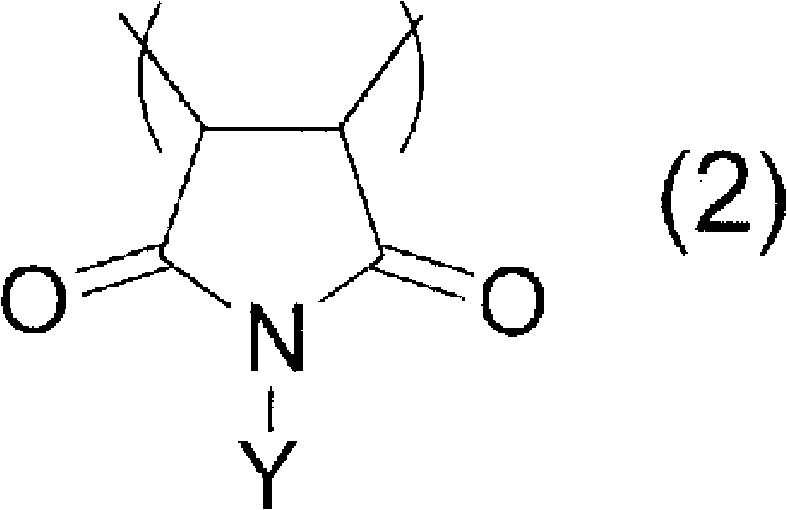
Photosensitive resin composition containing copolymer
A technology of photosensitive resin and composition, applied in the field of photosensitive resin composition, can solve the problems such as no indication of heat resistance, no description and no substitution, etc., and achieve reduced deformation, excellent heat resistance and solvent resistance, and excellent light resistance. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0090] Hereinafter, the present invention will be described in more detail based on examples and comparative examples, but the present invention is not limited to these examples.
[0091] [Measurement of weight-average molecular weight of polymers obtained from the following synthesis examples]
[0092] Device: GPC system manufactured by JASCO Co., Ltd.
[0093] Column: Shodex [registered trademark] KL-804L and 803L
[0094] Column oven: 40°C
[0095] Flow rate: 1ml / min
[0096] Eluent: tetrahydrofuran
[0097] [Synthesis of Polymer]
Synthetic example 1
[0099] Monoallyl isocyanuric acid 33g, N-cyclohexylmaleimide 15g, 1,4-di 88 g of alkanes were put into the flask, and the inside of the container was adjusted to 70°C to dissolve. Then, 2.4 g of 2,2'-azobis(isobutyrate) dimethyl and 1,4-bis A solution of 30 g of alkanes was added dropwise to the flask. Thereafter, it was heated to reflux for 8 hours, and then returned to room temperature, and the resulting solution was added to methanol to obtain 21 g of a polymer (copolymer) having a structural unit represented by the following formula (8) as a white powder. As a result of GPC analysis of the reaction product, the weight average molecular weight in terms of standard polystyrene was 8,400.
[0100]
Synthetic example 2
[0102] Monoallyl isocyanuric acid 13g, N-cyclohexylmaleimide 15g, maleimide 5g, 1,4-di Add 50 g of alkane into the flask and heat to reflux. Then, 1.6 g of 2,2'-azobis(isobutyrate) dimethyl and 1,4-bis A solution of 30 g of alkanes was added dropwise to the flask. Thereafter, it was heated to reflux for 10 hours, and then returned to room temperature, and the obtained solution was added to methanol to obtain 12 g of a polymer (copolymer) having a structural unit represented by the following formula (9) as a white powder. As a result of GPC analysis of the reaction product, the weight average molecular weight in terms of standard polystyrene was 12,900.
[0103]
PUM
| Property | Measurement | Unit |
|---|---|---|
| coating thickness | aaaaa | aaaaa |
| aperture size | aaaaa | aaaaa |
| coating thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com