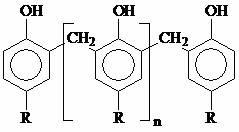
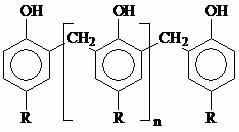
Halogen-free flame retardant containing linear phenolic aldehyde and polymer material containing halogen-free flame retardant
A kind of novolac, inorganic flame retardant technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-7
[0034] Put a certain amount of ABS resin at 180 0 After plasticizing on the double-roller mill of C, add each raw material in turn according to the formula (Table 1), blend evenly, and place the material in the mold after mixing, at 180 0 C’s plate vulcanizer is hot-pressed for 7-10 minutes, then cold-pressed at room temperature for 10 minutes, and finally the plate is molded and used for performance testing.
[0035] Table 1 Flame-retardant ABS blending formula / (weight parts)
[0036] Comparative example 1 Comparative example 2 Example 1 Example 2 Example 3 Example 4 Example 5 Example 6 Novolac: microencapsulated red phosphorus 1:1 3:2 3:2 3:2 2:3 1:1 Flame retardant percentage content / % 12 17 12 15 15 20 25 ABS 100 88 83 88 85 75 72 67 Novolac — — 6 7.2 9 9 8 12.5 Microencapsulated red phosphorus — 12 6 4.8 6 6 12 12.5 Aluminum hydroxide — — 5 — — — — — Nitrile ...
Embodiment 3
[0038] In Example 3 and Example 4, the ratio of novolac to microcapsule red phosphorus is 3:2, and when the total addition amount is 15%, the difference is that in Example 4, 10% nitrile rubber is used to replace part of the ABS. After the replacement, the impact strength of Example 4 was greatly improved, which was 3.76 times that of Example 3, but the tensile strength decreased by 13.6%, and the flame retardancy decreased from V-0 to V-1.
[0039] Examples 5 and 6 continue to increase the content of novolak and microencapsulated red phosphorus, the flame retardancy of the obtained material is further improved, and the notched impact strength of the obtained material is greatly improved after adding nitrile rubber.
[0040]
[0041] Table 2 Physical properties of flame retardant ABS
[0042] Test items Comparative example 1 Comparative example 2 Example 1 Example 2 Example 3 Example 4 Example 5 Example 6 Tensile strength (MPa) 39.2 38.7 37.1 ...
Embodiment 7
[0044] Mix 6 kg of novolac with 4 parts of red phosphorus powder and 0.1 kg of magnesium hydroxide evenly, and then add them to a twin-screw extruder with a temperature set at 150°C to extrude and granulate to obtain novolac compounded with red phosphorus. Halogen flame retardant A.
[0045] Mix 8.5 kg of ABS with 1.5 kg of the above-mentioned halogen-free flame retardant A, extrude and granulate on a twin-screw extruder at 220°C, and then inject the obtained flame-retardant ABS on an injection molding machine at 220°C to prepare samples. The properties of the obtained samples are shown in Table 3
[0046] Table 3 Example 7 sample properties
[0047] project Example 7 Tensile Strength (MPa) 38.2 Notched impact strength (KJ m -2 ) 3.8 Flame retardant (1.6mm) V-0
[0048] Table 4 Various performance test standards
[0049] project standard test Tensile Strength GB / T1040-2006 Notched impact strength GB / T1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| impact strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com