Catalyst for methanating coke oven gas and preparation method thereof
A coke oven gas and catalyst technology, applied in physical/chemical process catalysts, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problems of increased equipment investment, large catalyst consumption, and lack of provision, etc. problems, to achieve high activity, improved thermal stability, and high operating space velocity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
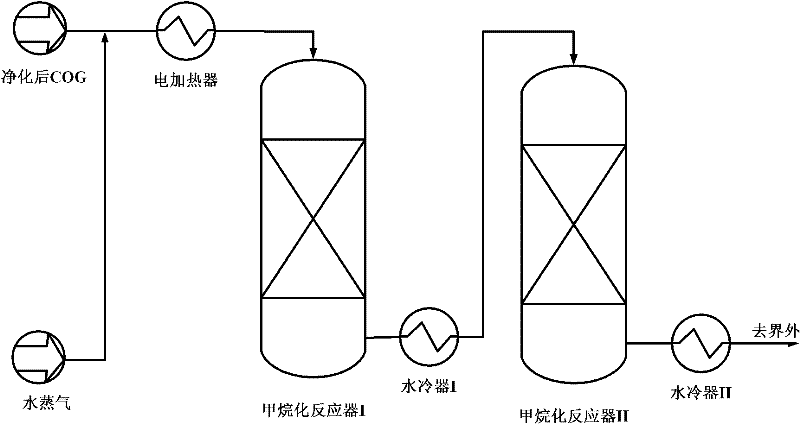
Image
Examples
Embodiment 1
[0025] 90g Na 2 CO 3 Dissolve in 350ml deionized water for later use. 198g Ni(NO 3 ) 2 ·6H 2 O, 31g La(NO 3 ) 3 ·6H 2 O was dissolved in 400ml deionized water and stirred evenly to form a solution 1, 78gMn(NO 3 ) 2 (50% content), 19g Zr(NO 3 ) 2 ·5H 2 O was dissolved in 200ml deionized water and stirred uniformly to form solution 2. 114g Al 2 o 3 Slowly add the powder to solution 1 and stir well. To this solution was added solution 2 followed by Na 2 CO 3 The solution allowed sufficient precipitation of the metal ions, and stirring was continued for 30 minutes. After washing and drying, the precipitate was dried at 120°C for 8 hours, and then the precipitate was calcined in a muffle furnace at 600°C for 4 hours, and a φ3×3mm cylindrical catalyst was obtained through a tableting process. The catalyst was reduced at 400° C. for 4 hours under a hydrogen atmosphere to obtain a final coke oven gas methanation catalyst.
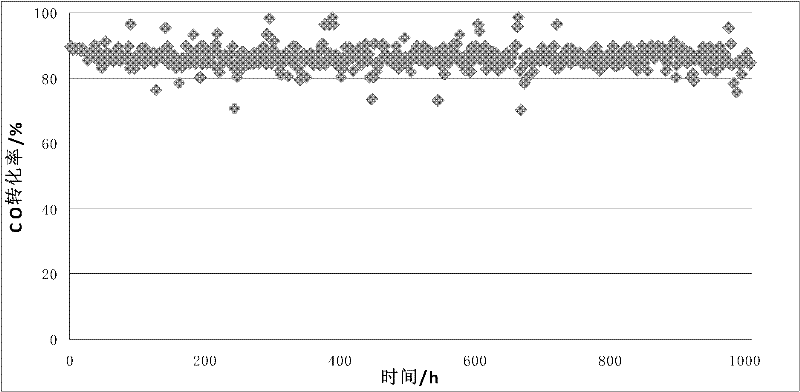
[0026] Activity test results: CO conversio...
Embodiment 2
[0028] 103g Na 2 CO 3 Dissolve in 350ml deionized water for later use. 200g Ni(NO 3 ) 2 ·6H 2 O was dissolved in 400ml deionized water and stirred evenly to form solution 1, 20g La(NO 3 ) 3 ·6H 2 O, 10g Sr(NO 3 ) 2 4H 2 O, 15g Zr(NO 3 ) 2 ·5H 2 O was dissolved in 100ml deionized water and stirred uniformly to form solution 2. 120g Al 2 o 3Slowly add the powder into solution 1 and stir evenly, add Na to the solution at the same time 2 CO 3 Solution and additive solution 2 fully precipitate metal ions, and continue to stir for 30 minutes. After washing and drying, the precipitate was dried at 120°C for 8 hours, and then the precipitate was calcined in a muffle furnace at 600°C for 4 hours, and a φ3×3mm cylindrical catalyst was obtained through a tableting process. The catalyst was reduced at 400° C. for 4 hours under a hydrogen atmosphere to obtain a final coke oven gas methanation catalyst.
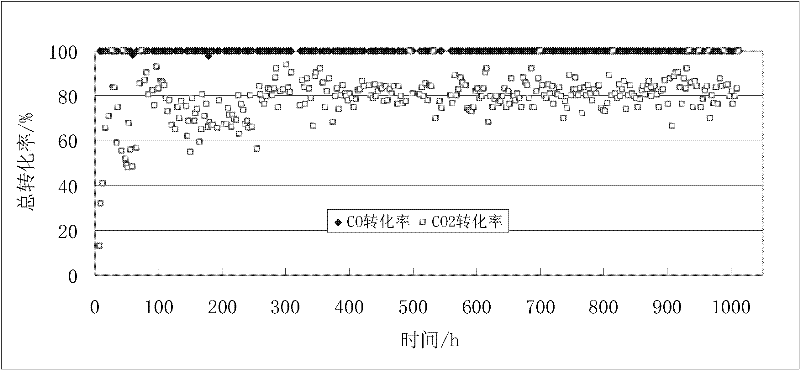
[0029] Activity test results: CO conversion rate is 84.17%, CH 4 T...
Embodiment 3
[0031] Dissolve 70g NaOH in 350ml deionized water for later use. 200g Ni(NO 3 ) 2 ·6H 2 O, 10g La(NO 3 ) 3 ·6H 2 O, 10g Zr(NO 3 ) 2 ·5H 2 O, 7g Ce(NO 3 ) 3 ·6H 2 O was dissolved in 400ml deionized water and stirred well. Slowly add 132g Al with stirring 2 o 3 Mix the powder well. NaOH solution was added to the solution to fully precipitate metal ions, and stirring was continued for 30 minutes. After washing and drying, the precipitate was dried at 120°C for 8 hours, and then the precipitate was calcined in a muffle furnace at 600°C for 4 hours, and a φ3×3mm cylindrical catalyst was obtained through a tableting process. The catalyst was reduced at 400° C. for 4 hours under a hydrogen atmosphere to obtain a final coke oven gas methanation catalyst.
[0032] Activity test results: CO conversion rate is 80.35%, CH 4 The selectivity is 77.56%; the conversion rate of CO under this condition is theoretically calculated to be 87.93%, and the CH 4 The selectivity is: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com