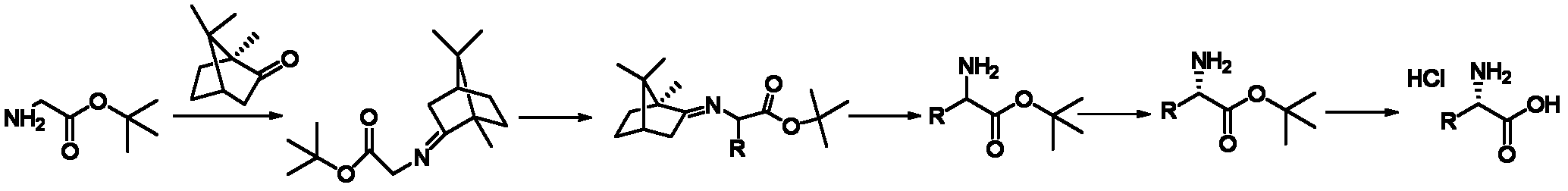
Method for preparing chiral alpha-alkyl substituted glycine hydrochloride
A technology of glycine hydrochloride and hydrocarbyl substitution, applied in the field of synthesis of chiral amino acid hydrochloride, can solve the problems of high energy consumption, unsuitable for large-scale production and high requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A kind of preparation L-allylglycine hydrochloride The method is characterized in that the specific preparation steps are as follows:
[0035] (1) Condensation: Add 470kg (1g / 6mL) of toluene, 90kg (1.0eq) of (S)-camphor, and 116kg (1.5eq) of tert-butyl glycine as the main raw material into a 1000L reactor. After stirring, add 1.26 dropwise. kg boron trifluoride diethyl ether (0.015eq), after dripping, the temperature is raised to 90±5℃ to react; after the reaction is completed, the temperature is lowered, the system is washed with sodium bicarbonate solution to pH=7-8, and the organic phase is concentrated to obtain the product 141kg, yield 90.0%, gas chromatography purity (GC): 96.8%;
[0036] (2) Substitution: Add 400kg of tetrahydrofuran (1g / 10mL) and 26.6kg (1.4eq) of potassium tert-butoxide to a 1000L reactor, cool to -10±2℃, and add the main raw materials dropwise 45kg of tetrahydrofuran (1g / 2mL) solution 125kg, stir for 0.5 hour, add 28.7kg (1.4eq) of 3-bromopropene...
Embodiment 2
[0042] A kind of preparation D-ethyl glycine hydrochloride The method is characterized in that the specific preparation steps are as follows:
[0043] (1) Condensation: Add 66kg (1g / 1mL) of n-heptane, (S) 100kg (1.0eq) of camphor, and 86kg (1.0eq) of the main raw material tert-butyl glycine to a 500L reactor. After stirring, add dropwise 932g (0.01eq) of boron trifluoride ether, after dripping, raise the temperature to 80±2℃ for reaction; after the reaction is completed, the temperature is lowered, the system is washed with sodium bicarbonate solution to pH=7~8, and the organic phase is concentrated to obtain the product 155kg, yield 89.0%, gas chromatography purity (GC) 96.5%;
[0044] (2) Replacement: Add 103kg (1g / 6mL) of 2-methyltetrahydrofuran (1g / 6mL) and 7.2kg (1.0eq) of sodium tert-butoxide to a 500L reactor, cool to -20~10℃, and add the main raw materials dropwise. 20kg of 2-methyltetrahydrofuran (1g / 4mL) solution, 90kg, stir for 0.3 hours, add 8.2kg (1.0eq) of bromoetha...
Embodiment 3
[0050] A kind of preparation L-benzylglycine hydrochloride The method is characterized in that the specific preparation steps are as follows:
[0051] (1) Condensation: Add 870kg xylene (1g / 10mL), (S) camphor 100kg (1.0eq), and 155kg (1.8eq) of tert-butyl glycine as the main raw material into a 2000L reactor. After stirring, add three 1.86kg (0.02eq) of boron fluoride ether, after dripping, raise the temperature to 110±2℃ to react; after the reaction is completed, the temperature is lowered, the system is washed with sodium bicarbonate solution to pH=7~8, the organic phase is concentrated to obtain the product 160kg; yield 91.8%, gas chromatography purity (GC) 96.0%;
[0052] (2) Substitution: Add 266kg of methyl tert-butyl ether (1g / 12mL) and 28kg of potassium carbonate (1.8eq) to a 1000L reactor, cool to 10±2℃, and add the main raw materials dropwise 30kg of methyl tert-butyl ether (1g / 6mL) solution 164kg, stir for 1 hour, add 34.8kg of benzyl bromide (1.8eq) dropwise, drip at ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com