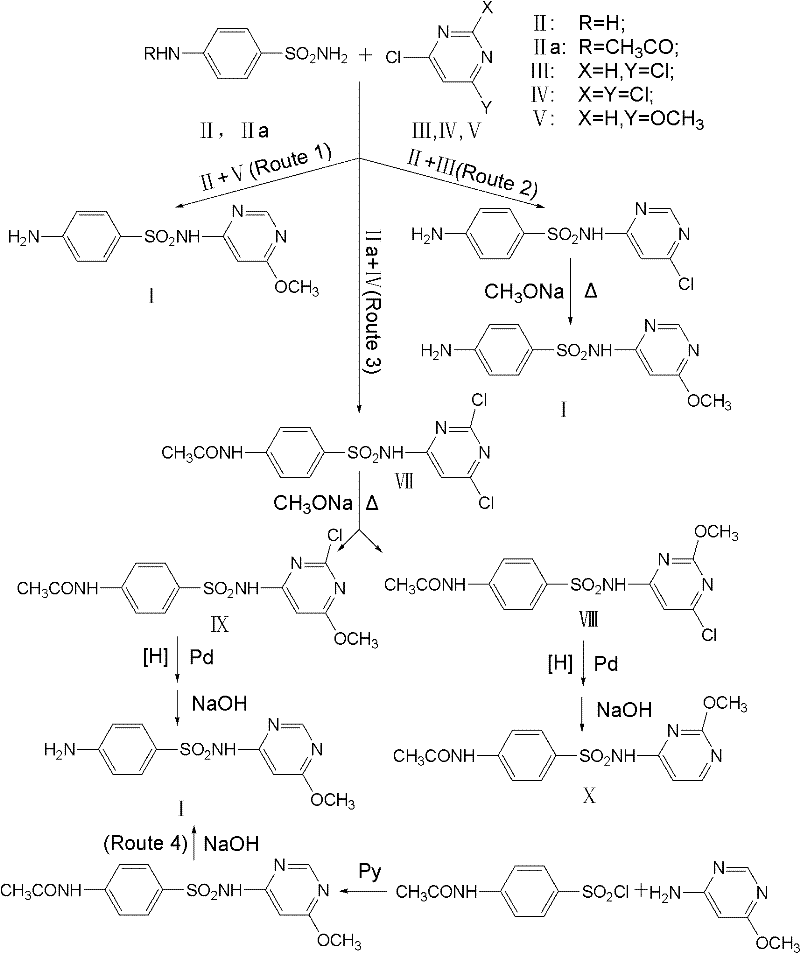
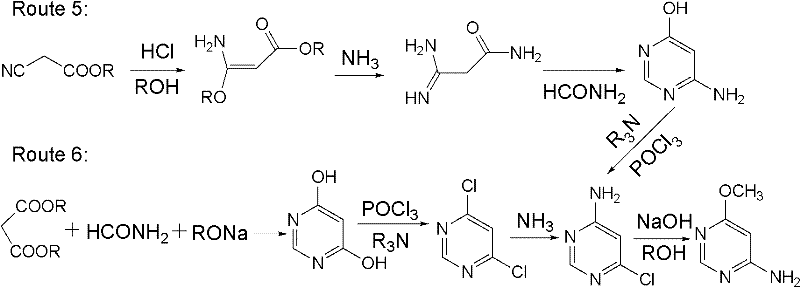
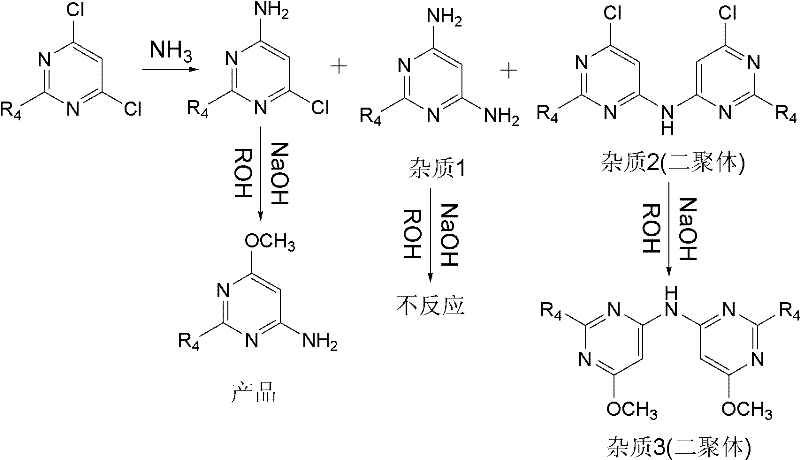
Preparation method for 4-amino-6-alkoxyl pyrimidine compounds
A technology for alkoxypyrimidine and amine compounds, which is applied in the field of preparation of 4-amino-6-alkoxypyrimidine compounds, can solve the problems of complicated operation, high content of impurity 3, low yield and the like, and achieves process simplification , High product yield, the effect of reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] In a 500ml four-necked bottle, it is equipped with a thermometer, a stirrer, a vent pipe, an exhaust gas absorption bottle, and water in the absorption bottle. Then add 250.0 g of water and 125.0 g of 4,6-dichloropyrimidine into the bottle, raise the internal temperature to 50-55°C in a hot water bath, replace the air in the kettle with ammonia gas twice, and feed ammonia gas under controlled flow rate, Stir the reaction, the exothermic reaction will cause the temperature of the system to rise, and the rate of passing ammonia should be such that the internal temperature of the reaction solution is 56-60°C, and there is basically no bubbles in the tail gas absorbing bottle. A total of 70g of ammonia is passed.
[0055] The reaction liquid was sampled for HPLC analysis, and the content of 4,6-dichloropyrimidine was 0.07156% (Area%), the content of 4-amino-6-chloropyrimidine was 98.5369% (Area%), and the content of impurity 2 was 0.6961% (Area%). Stop the reaction, lower t...
Embodiment 2~7
[0058] Change the ammonia total amount that feeds, other conditions are constant, repeat the operation in embodiment 1, gained result is as follows table:
[0059]
Embodiment 8~11
[0061] Ammonia total amount is fixed at 64.2g, changes the consumption of water, other conditions are constant, repeats the operation in embodiment 1, gained result is as follows:
[0062]
[0063]
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com