Natural L-alpha-glycerophosphocholine (GPC) and preparation method thereof
A glycerol phosphatidylcholine, a natural technology, applied in the direction of phosphorus organic compounds, can solve the problems of difficult separation and removal, complicated separation process of toxic mercury salts, and low total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
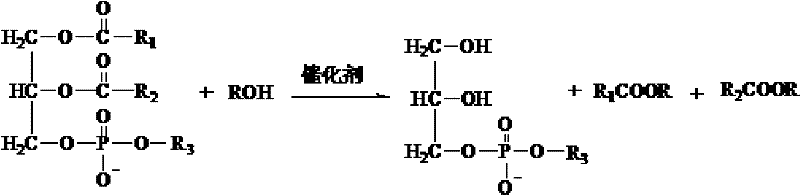
[0028] The preparation method of natural L-alpha-glycerophosphatidylcholine of the present invention specifically comprises the steps:
[0029] 1) Add the formulated amount of lecithin into the three-necked flask with condensing reflux, and dissolve it with the formulated amount of low-carbon alcohol, then place the three-necked flask in a collector-type constant temperature heating magnetic stirrer and heat it to 20 ° C ~ 100 ° C .
[0030] 2) After the temperature of the reaction liquid is constant, add the catalyst in the formula amount and stir for 2h-15h.
[0031] 3) Carry out the vacuum rotary evaporation of the mixed liquid after the reaction in step 2), the evaporation temperature is 40°C-60°C, the purpose is to remove the unreacted lower alcohol and catalyst therein. Dissolve the product obtained by vacuum rotary evaporation with 1-2 times the volume of low-carbon alcohol, and then use 2-10 times the volume of non-polar solvent for precipitation. After the precipitat...
Embodiment 1
[0038] Add 2g of soybean lecithin with a phosphatidylcholine content of 90% to a three-neck flask with reflux, dissolve it with 20mL of anhydrous methanol, heat it to 60°C in a constant temperature water bath, and then add 0.01mol of low boiling point (boiling point is 30 ~ 90 ℃) catalyst, stirred and reacted for 6 hours.
[0039] After the reaction, use a vacuum rotary evaporator to evaporate the reacted mixed solution at 60°C; dissolve the oily precipitate at the bottom of the container with 5 mL of anhydrous methanol, and add 20 mL of ether for precipitation. Discard the upper part after the precipitation is complete. Clear liquid, then dissolved with methanol and precipitated with ether three times to obtain the colorless oily product L-α-glycerol phosphatidylcholine, and vacuum-dried the obtained colorless oily product in the presence of phosphorus pentoxide , and finally get the GPC. Low-carbon alcohols and catalysts obtained by vacuum rotary evaporation can be recovere...
Embodiment 2
[0045] The difference between this example and Example 1 is that a nonionic organic amine with a high boiling point (90-220° C.) is selected as a catalyst in the reaction stage. After the reaction was completed, the temperature for vacuum rotary evaporation was 40°C.
[0046] Under the condition that the various conditions of this embodiment are constant, the types of catalysts used are different, and the yields of GPC are also different. The specific results are shown in Table 2.
[0047] The yield of GPC under table 2 high boiling point catalyst
[0048] catalyst type
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com