Selective ionic liquid, synthesis method, and application thereof
An ionic liquid and selective technology, applied in separation methods, chemical instruments and methods, and separation of dispersed particles, can solve the problems of rodent learning ability and exercise ability, liver toxicity, reproductive toxicity, etc., and meet the requirements of the reaction conditions. High, easy to operate, simple installation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
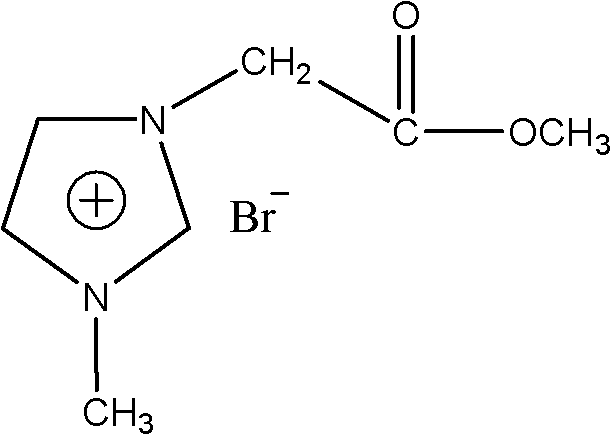
[0026] 1-Acetoxymethyl-3-methylimidazolium bromide ([CH 2 Synthesis of COOMeMIM]Br)
[0027]
[0028] in N 2 Under protection, using acetonitrile as a solvent, add 18.5mL (0.2mol) of methyl bromoacetate to the reaction vessel, slowly add 15.9mL (0.2mol) of N-methylimidazole dropwise into the reaction vessel, and make it react with methyl bromoacetate The ester molar ratio is 1:1~1:1.5. First, stir continuously at room temperature to fully mix the two, and then heat to reflux for 12 hours to obtain the crude bromo-1-acetate-3-methylimidazolium ionic liquid , separated with a separatory funnel while hot, washed the crude product several times with ethyl acetate, distilled off the solvent and ethyl acetate under reduced pressure, and dried in vacuo to obtain a white solid that is the purified ionic liquid intermediate product 1-acetate-3 - Methylimidazolium bromide. The obtained product was confirmed to be the compound described in the title by infrared spectrum and nuclear...
Embodiment 2
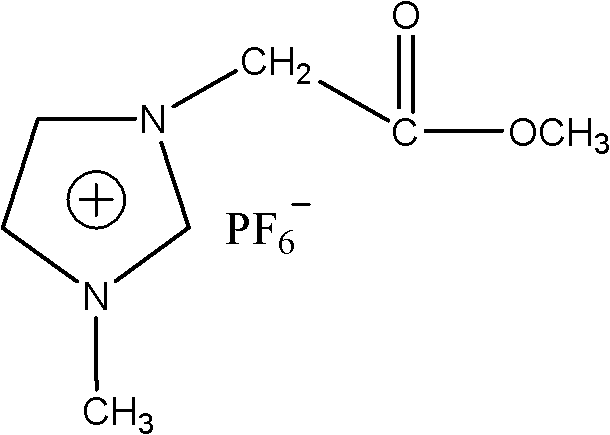
[0030] 1-Acetoxymethyl-3-methylimidazolium hexafluorophosphate ([CH 2 COOMeMIM]PF 6 )Synthesis
[0031]
[0032] Dissolve potassium hexafluorophosphate and purified 1-acetoxymethyl-3-methylimidazolium bromide salt with appropriate amount of distilled water respectively, mix the two to control the molar ratio of 1:1~1:1.2, and stir at room temperature for 12 hours , filtered and washed the product several times, and dried in vacuum to obtain the final product of the ionic liquid, 1-acetate-3-methylimidazolium hexafluorophosphate. The obtained product was confirmed to be the compound described in the title by infrared spectrum and nuclear magnetic resonance spectrum analysis, and the data content is shown in title five.
Embodiment 3
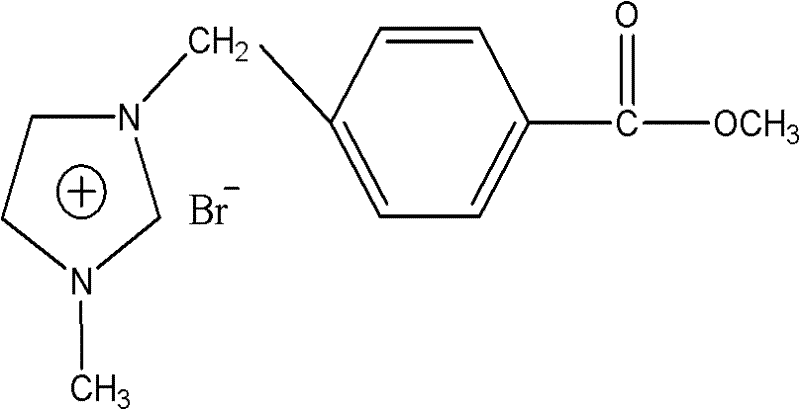
[0034] 1-methylbenzoate-3-methylimidazolium bromide ([CH 2 C6H 4 Synthesis of (p-COOMe)MIM]Br)
[0035]
[0036] in N 2 Under protection, using acetonitrile as a solvent, add 13.75g (0.06mol) of methyl p-bromomethylbenzoate to the reaction vessel, slowly add 4mL (0.05mol) of N-methylimidazole dropwise into the reaction vessel, keep stirring and heating After reflux reaction for 12 hours, the system was divided into upper and lower layers after the reaction was completed, and separated with a separatory funnel to obtain the crude product of 1-p-methylbenzoic acid methyl-3-methylimidazolium bromide salt ionic liquid in the lower layer, which was washed with ethyl acetate for several The solvent and ethyl acetate were removed by extraction and separation under reduced pressure, and the white solid was obtained by vacuum drying, that is, the purified ionic liquid intermediate product 1-p-methylbenzoate-3-methylimidazolium bromide. The obtained product was confirmed to be the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com