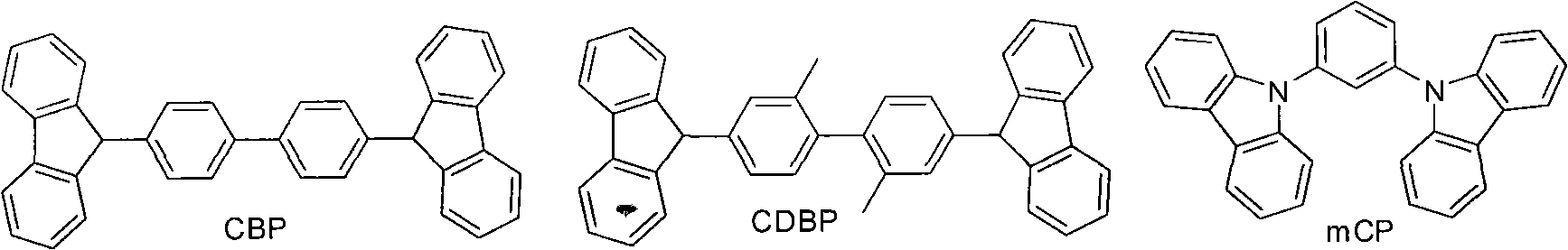
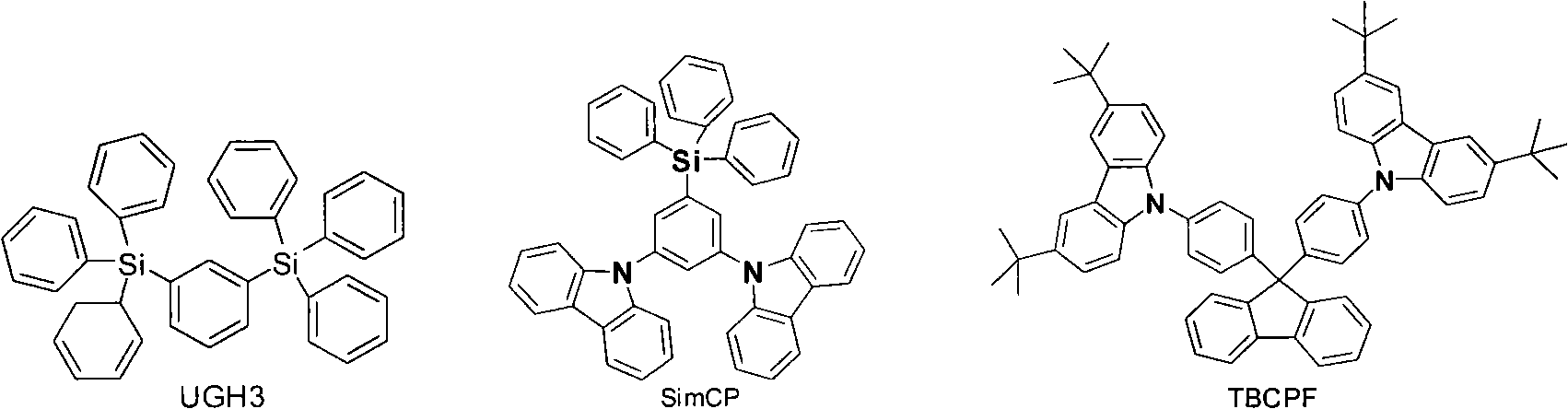
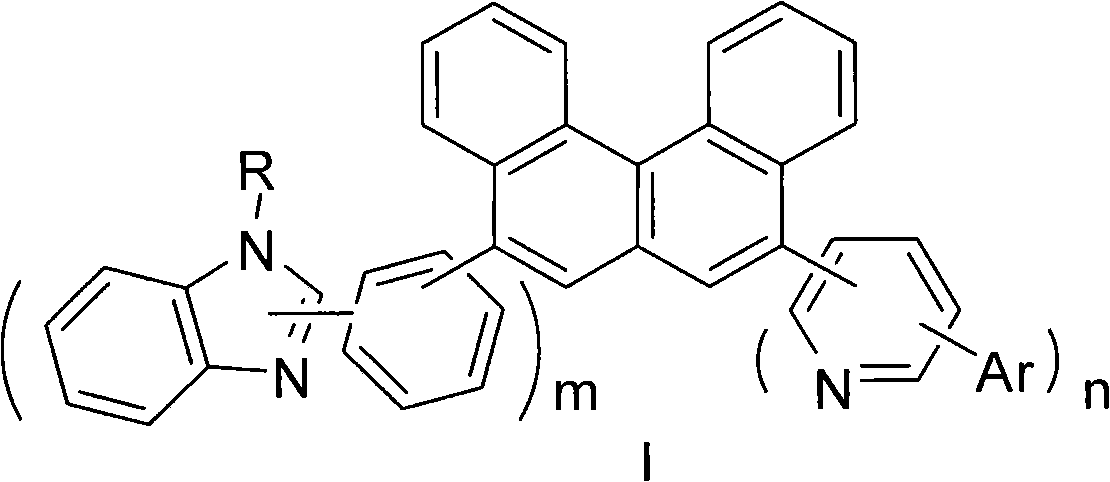
Benzophenanthrene compound containing benzoglioxaline group and application thereof
A compound and organic technology, applied in the field of new organic materials, can solve problems such as inhibition of endothermic energy transfer process, short life of blue phosphorescent devices, and reduced device efficiency, so as to achieve high electron transport performance, improve electron transport performance, and film formation good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation of embodiment 1 compound 1-1
[0040]
[0041] 11.8g (0.10mmol) of benzimidazole was dissolved in 10.1mL of triethylamine, 28.1g (0.10mmol) of isobromoiodobenzene was added dropwise at zero temperature, stirred at room temperature for 30 minutes, then heated to 70-80°C and stirred for 2 hours. The reaction was quenched with water, extracted with dichloromethane, dried over magnesium sulfate, and recrystallized from methanol to obtain 23.8 g of compound A, which was dissolved in dry THF, and n-butyl was added dropwise at -80°C, stirred for 15 minutes, and then three Isopropyl borate. Hydrolyze, adjust the pH to neutrality and precipitate compound B.20.2g. The coupling reaction between B and 5,8-dibromobenzo[c]phenanthrene 1:1 was carried out to obtain intermediate C, which was reacted with compound E to obtain compound 1-1. It was purified by column chromatography, and the eluent was petroleum ether:dichloromethane=2:1. MS (m / e): 573, elemental anal...
Embodiment 2-30
[0046] Examples 2-30 are all similar to Example 1, and the target product can be obtained by reacting intermediate C with different aryl-substituted pyridine boronic acids. The details are as follows:
Embodiment 2
[0047] The synthesis of embodiment 2 compound 1-2
[0048] Using intermediate C and 6-phenyl-2-pyridineboronic acid as raw materials, compound 1-2 is obtained. MS (m / e): 573, elemental analysis (C 42 h 27 N 3 ): theoretical value C: 87.93%, H: 4.74%, N: 7.32%; measured value C: 87.90%, H: 4.81%, N: 7.29%. Yield 61.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com