8-pyrazole substituted xanthine A2B adenosine receptor antagonist and synthesis method and application thereof
An adenosine receptor and a synthesis method technology, applied in the field of drug synthesis, can solve the problems of complex meta-trifluoromethylbenzyl synthesis conditions, severe reaction conditions, long reaction time and the like, and achieve easy industrial production, few reaction steps, Yield-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] R 1 , R 2 for hydrogen, R 3 For o-nitrobenzylpyrazole—synthesis of 8-(o-nitrobenzyl-1H-pyrazole)-xanthine
[0070] (1) Synthetic raw material o-nitrobenzyl-1H-pyrazole-4-carboxylic acid
[0071] ①Synthesis of ethyl pyrazole-4-carboxylate
[0072]
[0073] In a 250mL reaction flask equipped with a stirrer, a thermometer, a dropping funnel and a fractionating column, add 0.12mol of triethyl orthoformate, 0.10mol of ethyl cyanoethyl ester, 0.4g of acetic anhydride and 0.1g of a zinc chloride catalyst, and stir , reflux at 110°C for 1 hour, continue to heat up to 132°C, and distill off the low boiling point substances generated during the reaction from the top of the fractionating column; the reaction solution is cooled to precipitate crystals, filtered and washed with ethanol to obtain light yellow crystalline ethoxymethylene Ethyl cyanoacetate, Mp: 50~51°C; 0.1mol ethoxymethylene ethyl cyanoacetate and 0.10mol hydrazine hydrate were stirred in 400mL ethanol for 12h...
Embodiment 2
[0081] R 1 , R 2 for hydrogen, R 3 For m-nitrobenzylpyrazole—synthesis of 8-(m-nitrobenzyl-1H-pyrazole)-xanthine
[0082]
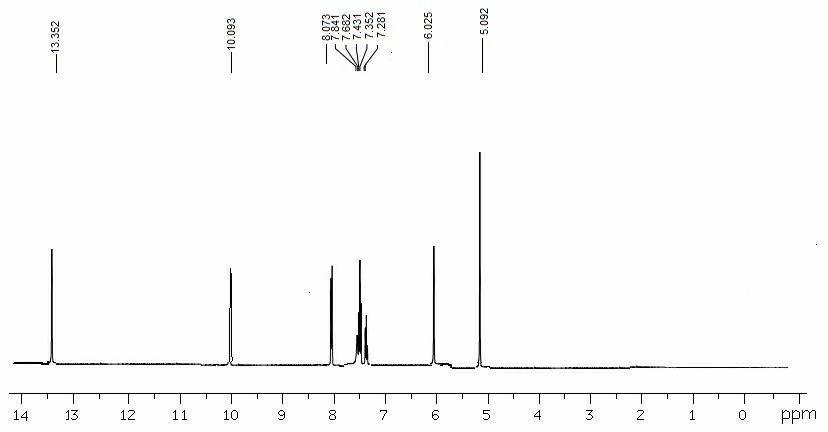
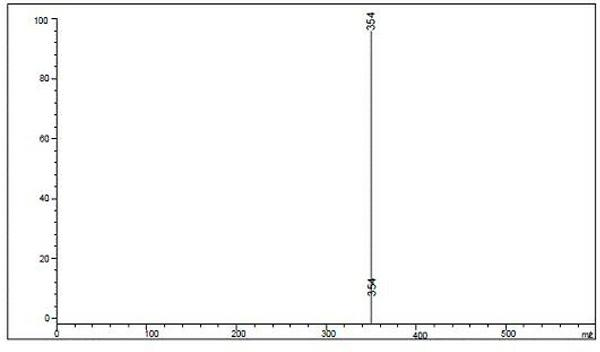
[0083] Dissolve 1.0mmol of m-nitrobenzyl-4-pyrazolecarboxylic acid and 1.0mmol of carbodiimide hydrochloride in 22mL of methanol, add 1.0mmol of 5,6-diaminouracil in 5 times under stirring, and stir for 12 hours , filter, remove solvent, wash with water, dissolve it in 15mL methanol and 15mL sodium hydroxide solution, react in microwave (360 W) for 30 min, cool to room temperature, acidify to pH with 6mol / L hydrochloric acid in ice bath =4, 8-(m-nitrobenzyl-1H-pyrazole)-xanthine was precipitated as a yellow solid, mass: 31.5 g, yield 89.3%., Mp: 196-198°C, purity: >95%. 1 H NMR (DMSO-d6): 13.39 (s,1H), 10.01(s,1H), 7.99-8.03 (m,2H), 7.81 (s,1H),7.62 (s,1H) 7.45-7.40 (m, 2H), 6.08 (s,1H), 5.01 (s, 2H) (eg image 3 ); LC-MS(m / z): 354 [M+] (eg Figure 4 ).
Embodiment 3
[0085] R 1 , R 2 for hydrogen, R 3 For p-nitrobenzylpyrazole—synthesis of 8-(p-nitrobenzyl-1H-pyrazole)-xanthine
[0086]
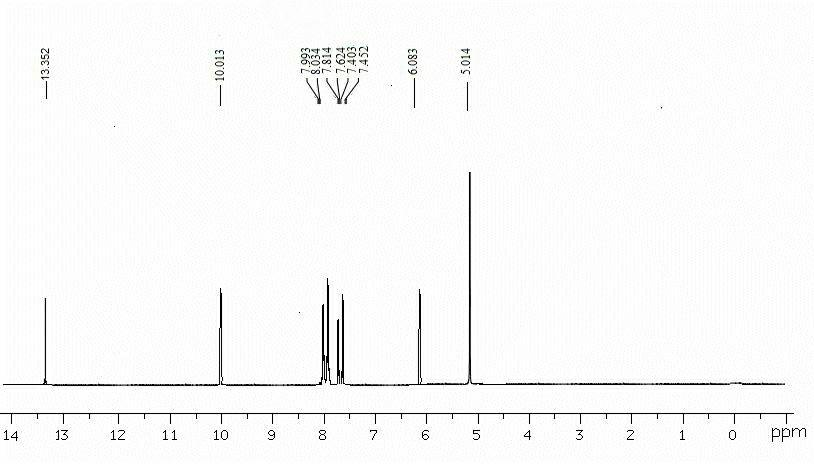
[0087] Dissolve 1.0mmol p-nitrobenzyl-4-pyrazolecarboxylic acid and 1.0mmol carbodiimide hydrochloride in 22mL methanol, add 1.0mmol 5,6-diaminouracil in 5 times under stirring, and stir for 10 hours , filter, remove solvent, wash with water, dissolve it in 15mL methanol and 15mL sodium hydroxide solution, react in microwave (350 W) for 20 min, cool to room temperature, acidify to pH with 6mol / L hydrochloric acid in ice bath =2, 8-(p-nitrobenzyl-1H-pyrazole)-xanthine was precipitated as a light yellow solid, mass: 28.4g, yield 82.8%, Mp: 208-210°C, purity: 97.4%. 1 H NMR (DMSO-d6): 13.39 (s,1H), 10.02(s,1H), 8.07(m,2H) ,7.61 (s, 1H),7.32 (m,2H), 7.43 (s,1H), 6.08 (s,1H), 5.01 (s,2H) (eg Figure 5 ); LC-MS(m / z):354 [M+] (eg Figure 6 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com