Preparation method of super-molecule metal gel
A metal gel and supramolecular technology, applied in zinc organic compounds, cadmium organic compounds, lead organic compounds, etc., can solve problems such as insufficient coordination bonds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1. Preparation of Gel G1
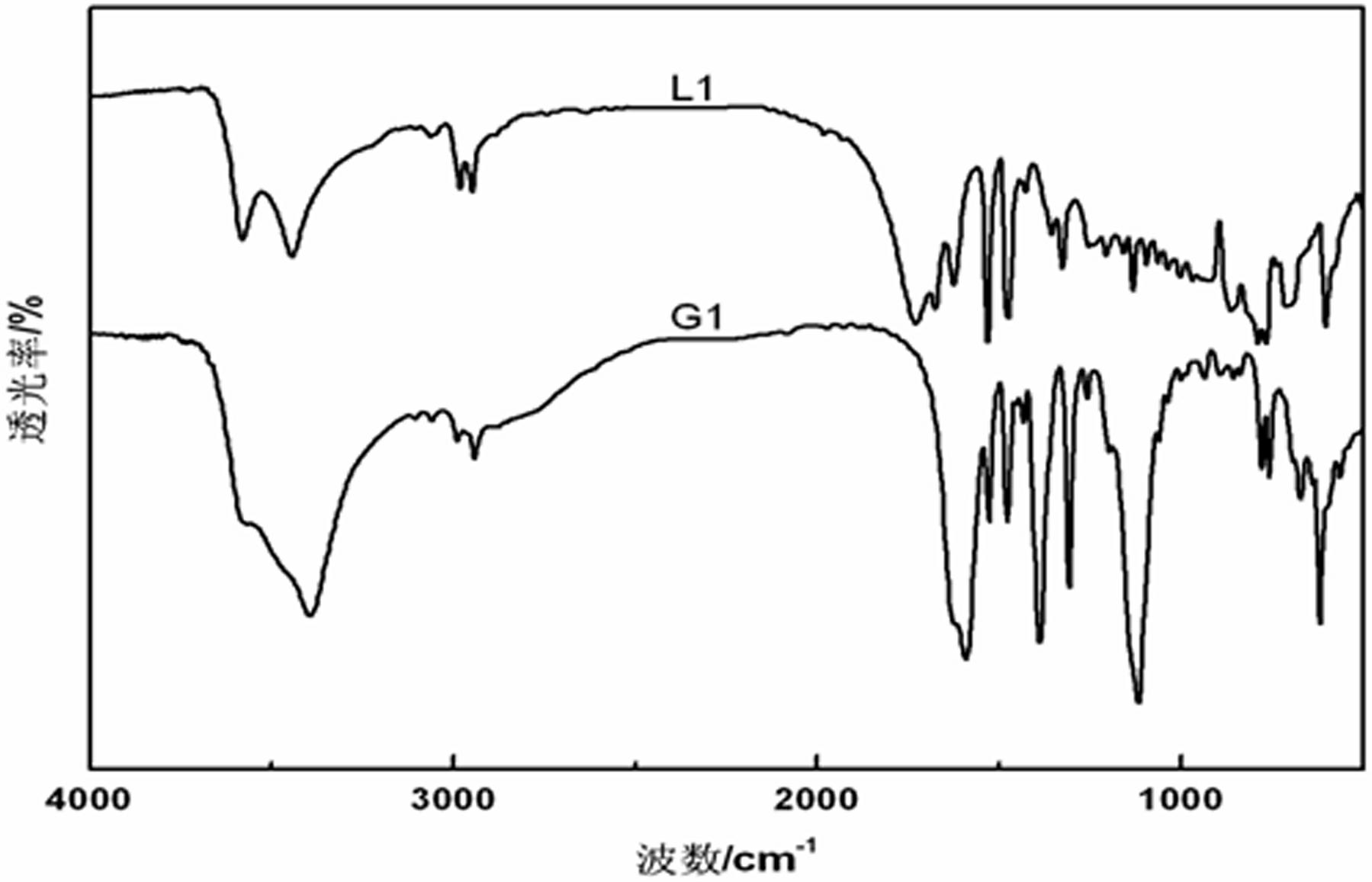
[0023] (1) Preparation of compound L1 (N,N'-dicarboxymethyl-2-ethylbenzimidazole):
[0024] Take 4.00g (42mmol) of chloroacetic acid in a three-necked round bottom flask equipped with a reflux condenser, and adjust the pH to 8~10 with NaOH solution, then add 3.49g (14mmol) of 2-ethylbenzimidazole and heat to reflux During the period, the pH value was continuously adjusted to maintain the pH value of the system at 8~10, until the pH value was stable, then continue to reflux for 30 minutes, cool, and add concentrated hydrochloric acid to adjust the pH=2~3, and a large amount of white precipitate was formed. Let it stand overnight, filter with suction, wash with distilled water and acetone 3 times, and then recrystallize with water to obtain a white solid.
[0025] Melting point: 279~281°C, yield: 70%. 1 H NMR (400MHz D 2 O, ppm) δ: 7.45~7.56 (m, 4H, ), 4.65~5.01 (d, 4H), 3.03~3.05 (d, 2H), 1.10~1.14 (t, 3H), IR (KBr) v: 1730 (C=O), 1676 (C=O)...
Embodiment 2
[0028] Example 2. Preparation of Gel G2
[0029] (1) Preparation of compound L2 (N,N'-Dicarboxymethyl-2-propylbenzimidazole):
[0030] Take 4.00g (42mmol) of chloroacetic acid in a three-necked round bottom flask equipped with a reflux condenser, and adjust the pH value to 8~10 with NaOH solution, then add 3.86g (14mmol) of 2-propylbenzimidazole and heat to reflux During the period, adjust the pH value continuously to keep the pH value of the system at 8~10 until the pH value is stable, then continue to reflux for 30min, cool, add concentrated hydrochloric acid to adjust the pH=2~3, a large amount of white precipitate is formed. Let it stand overnight, filter with suction, wash with distilled water and acetone 3 times, and then recrystallize with water to obtain a white solid.
[0031] Melting point: 285~286 o C yield: 66%. 1 H NMR (400MHz D 2 O, ppm) δ: 7.46~7.57 (m, 4H), 4.56~4.75 (d, 4H), 1.57~1.63 (m 2H), 2.99~3.03 (m, 2H), 0.85~0.90 (m, 3H), IR(KBr); IR (KBr) v: 1749 (C=O), 1...
Embodiment 3
[0034] Example 3. Preparation of Gel G1
[0035] (1) Preparation of compound L1 (N,N'-Dicarboxymethyl-2-propylbenzimidazole): same as in Example 1.
[0036] (2) Preparation of gel G1
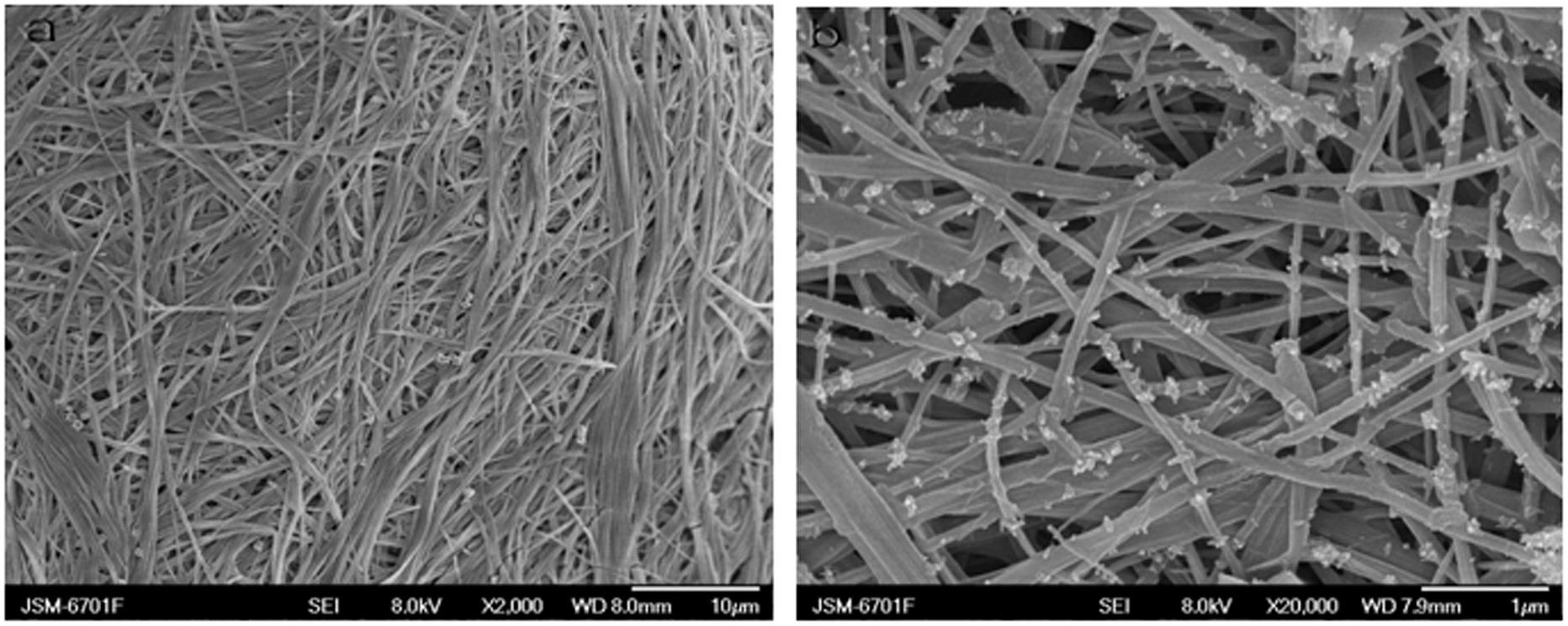
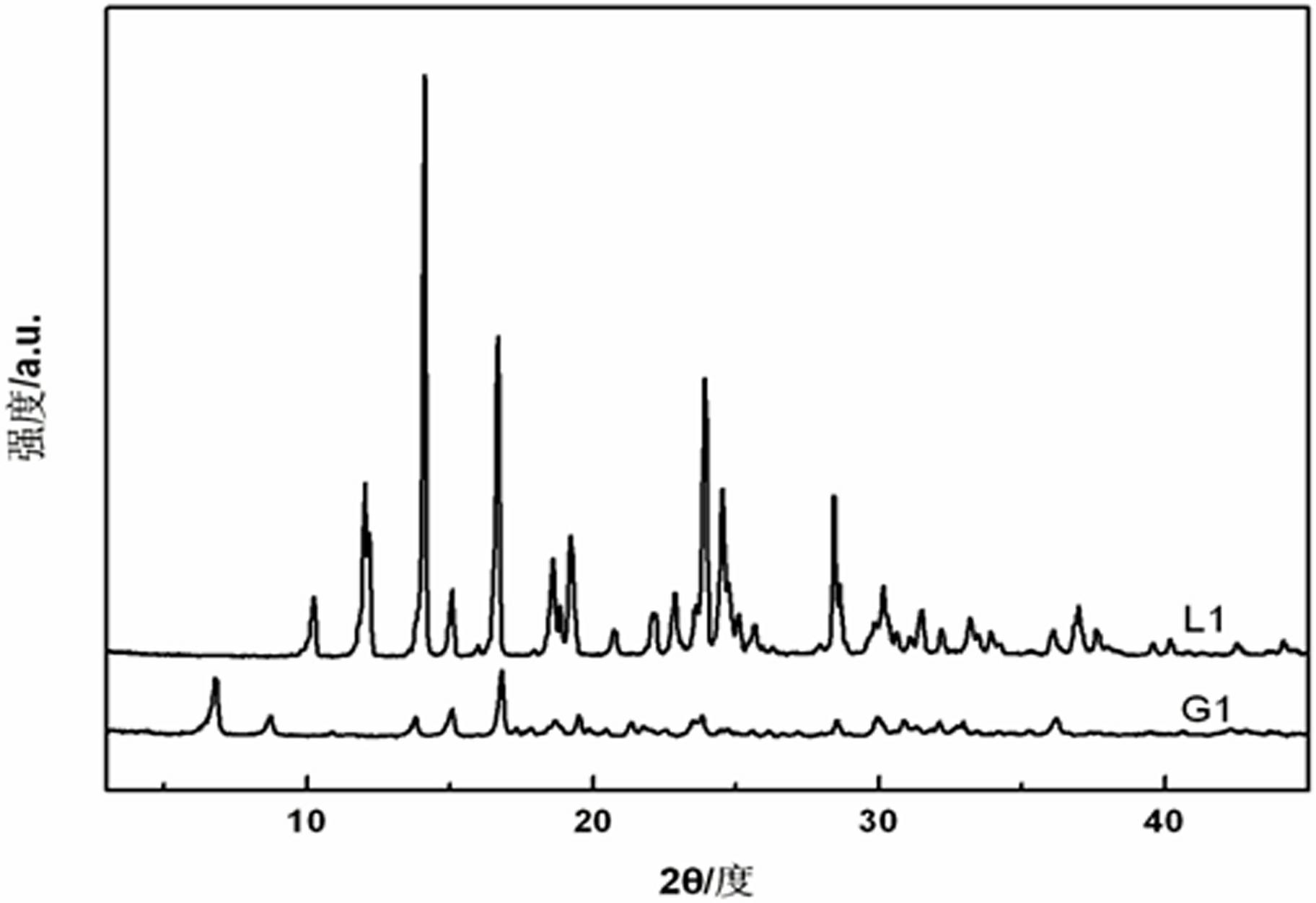
[0037] Mix compound L1 and copper sulfate in a ratio of 1:1 and add to H 2 O / DMSO mixed solution (H 2 The volume ratio of O to DMSO is 2:1). The white fibrous gel G1 is formed by ultrasonic treatment at 40 kHz for 6 seconds and placed at room temperature. The formed gel is heated to return to the solution state.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com