Pregnane glycoside compounds with orthoester groups and applications thereof
A technology of pregnane glycosides and compounds is applied to a class of pregnane glycosides containing orthoester groups and the fields of their uses, which can solve the problems of increasing the incidence of tumors, increasing infection of pathogenic microorganisms, and decreasing immune function. , to achieve the effect of weakening liver damage, reducing the incidence and reducing the severity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Preparation of Pregnane Glycosides Containing Orthoester Groups
[0056] (1) Preparation of Compounds P-1, P-2, P-5, P-6, P-7, P-3 and P-4
[0057] The pulverized and dried Persica sinensis medicinal material (15kg) was reflux extracted three times with 95% ethanol (30L × 3), each time for 2 hours, the extract was concentrated under reduced pressure to obtain ethanol extract, and about 2L of water was added to suspend it, followed by chloroform and normal Butanol extraction was carried out to obtain chloroform part extract (762.5 g) and n-butanol part extract (75.2 g). The chloroform part was separated by silica gel (200 mesh) column chromatography, and eluted with petroleum ether-acetone gradient (volume ratio was 5:1, 2:1, 1:1, 0:1 in turn) to obtain fraction Fr1 (601.3g) , Fr2 (15.8g), Fr3 (48.2g) and Fr4 (3.6g). Fr3 reversed phase C 18 Column chromatography, eluted with methanol-water gradient (volume ratio is 1:1, 2:1, 3:1, 4:1 and 1:0 in turn, to obta...
Embodiment 2
[0076]The in vitro immunosuppressive activity test of embodiment 2 compound P-1 to P-11
[0077] Toxicity evaluation of lymphocytes: mice were sacrificed by spinal method, their spleens were aseptically removed, ground to make a single cell suspension, red blood cells were removed with MTT solution (10% SDS, 50% DMF), and RPMI containing 10% FBS -1640 medium to adjust the cell concentration to 5×10 5 pieces / ml. Add 5×10 to 96-well plate 5 Cell suspension, 200μl RPMI-1640 culture medium and appropriate concentration of the sample to be tested, placed in 37C, 5% CO 2 Cultivate in the incubator for 48 hours, and add 18 μl of 5 mg / ml MTT to each well 5 hours before the end of the culture. At the end of the culture, add 90 μl of MTT solution to each well, place it in the incubator for 6-7 hours, and measure the OD570 value at 570 nm with a microplate reader.
[0078] Lymphocyte proliferation experiment: 5×10 5 Fresh spleen cells were stored at 37C, 5% CO 2 After conditioned c...
Embodiment 3
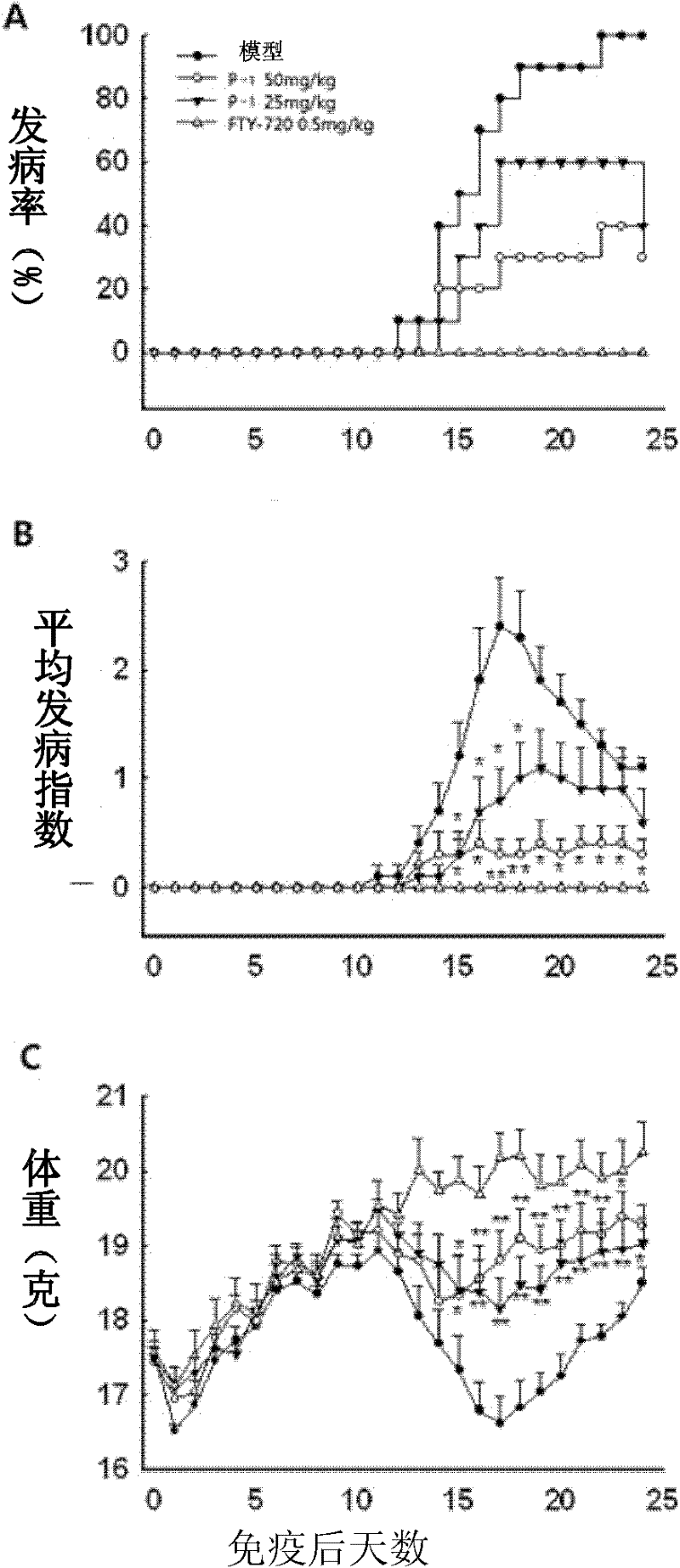
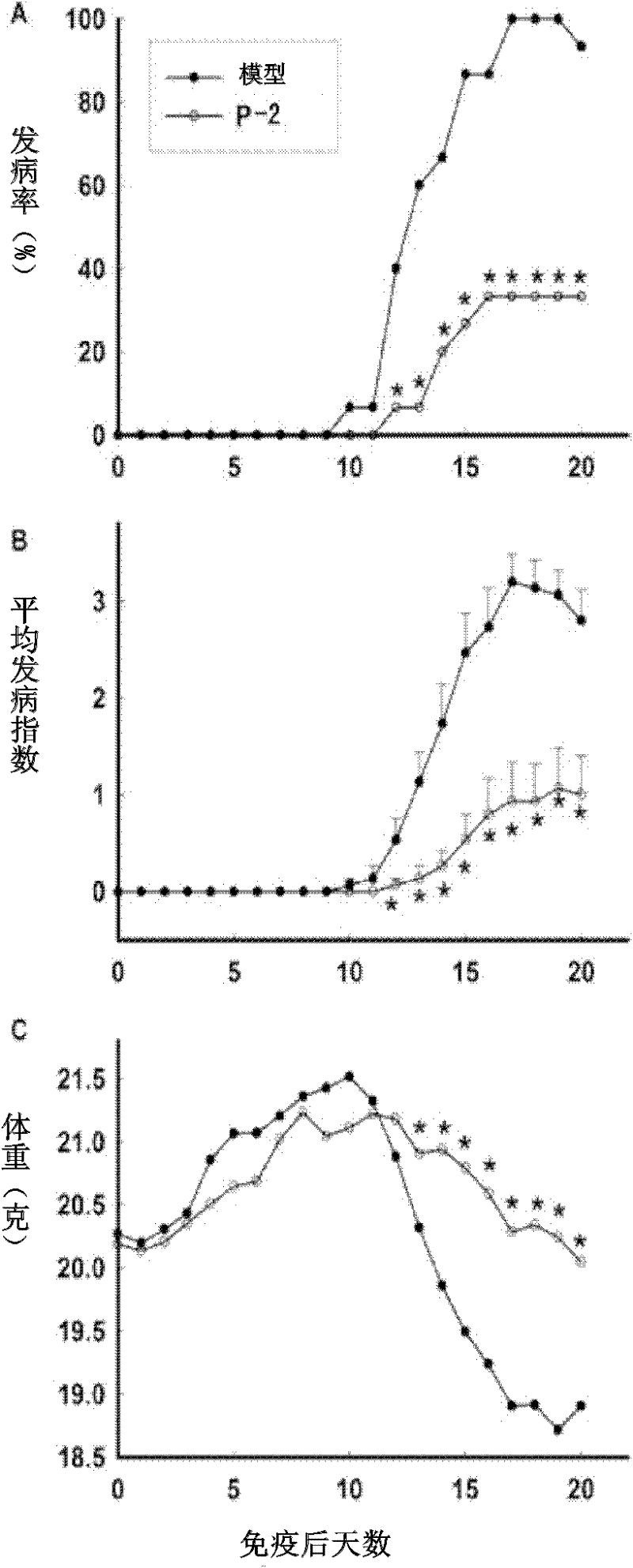
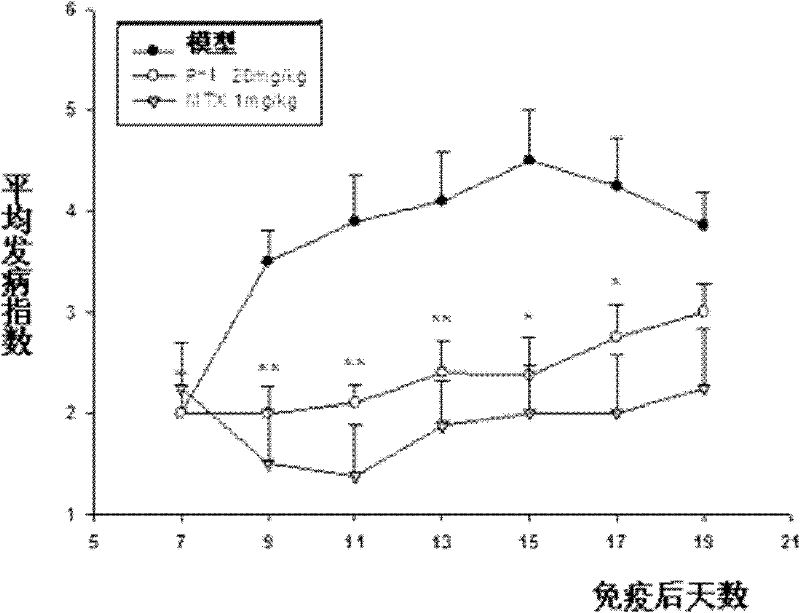
[0084] Example 3 Therapeutic effect of compounds P-1 and P-2 on animal models of autoimmune meningitis
[0085] C57BL / 6 female mice were immunized with MOG35-55 peptide to establish an acutely induced experimental autoimmune meningitis (EAE) model, and an equal volume of P-1 (50mg / kg / day, 25mg / kg / day) or P-2 (10mg / kg / day), positive control drug choose a new type of immunosuppressant FTY720 (chemical name 2-amino-2[2- (4-octylphenyl)ethyl]-1,3-propanediol hydrochloride, 2-amino-2[2-(4-octylphenyl)ethyl-1]-1,3-propanediol hydrochloride) (0.5mg / kg / day) or solvent control. Every day, each mouse was scored according to the established standard (0 points: no signs of disease; 1 point: weakness of tail or hind limbs; 2 points: weakness of tail and hind limbs; 3 points: partial paralysis of hind limbs; 4 points: complete paralysis of hind limbs; 5 points points: in moribund state or dead) and weighed.
[0086] The result is as figure 1 , figure 2 As shown (A: incidence rate of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com