Production method for hard butter
A technology of stearin and stearic acid, applied in dairy products, butter extraction, fat production, etc., can solve the problems of long working hours and high process costs, and achieve reduced working hours, reduced process costs, improved working time and process The effect of cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0036] Effects of high oleic acid content sunflower oil and Ethyl stearate is transesterified in order to obtain a fat-based material with an SOS content of at least 25 w / w%. The resulting material was distilled at 0.001 mbar and 230° C. to remove ethyl esters and have at least 98% triacylglycerol (TAG).
[0037] 1 kg of fat-based material obtained via transesterification was mixed with 4 kg of acetone and dissolved thoroughly, the container was closed with a stopper so as not to evaporate the acetone. Firstly, the mixture solution was subjected to high melting point separation and partial crystallization in a double-jacketed reactor. The mixture solution was stirred at 25°C and 30 rpm for 3 hours, followed by vacuum filtration, thereby separating into solid and liquid.
[0038] Filtered liquid fat was obtained with a yield of at least 98%, and the liquid was returned to the double-jacketed reactor and dissolved thoroughly at 60°C to collect the mid-melting point fat. To se...
example 2
[0040] As in Example 1, in the presence of the sn-1,3-specific enzyme Lipozyme RMIM (an immobilized sn-1,3-specific lipase from Rhizomucor miehei) on high oleic acid content sunflower The oil and ethyl stearate are transesterified so that the SOS content is at least 25 w / w%. Synthetic fats are distilled at 0.001 mbar and 230° C. to remove ethyl esters and have at least 98% triacylglycerols (TAG).
[0041] 1 kg of the fat-based substance obtained via transesterification was mixed with 4 kg of n-hexane and dissolved thoroughly, the container was closed with a stopper so as not to evaporate the n-hexane. Firstly, the solution was subjected to high melting point separation and partial crystallization in a double-jacketed reactor. The mixture solution was stirred at 10° C. and 30 rpm for 3 hours, followed by vacuum filtration, thereby separating into solid and liquid.
[0042] Filtered liquid fat was obtained with a yield of at least 98%, and the liquid was returned to the double...
experiment example 1
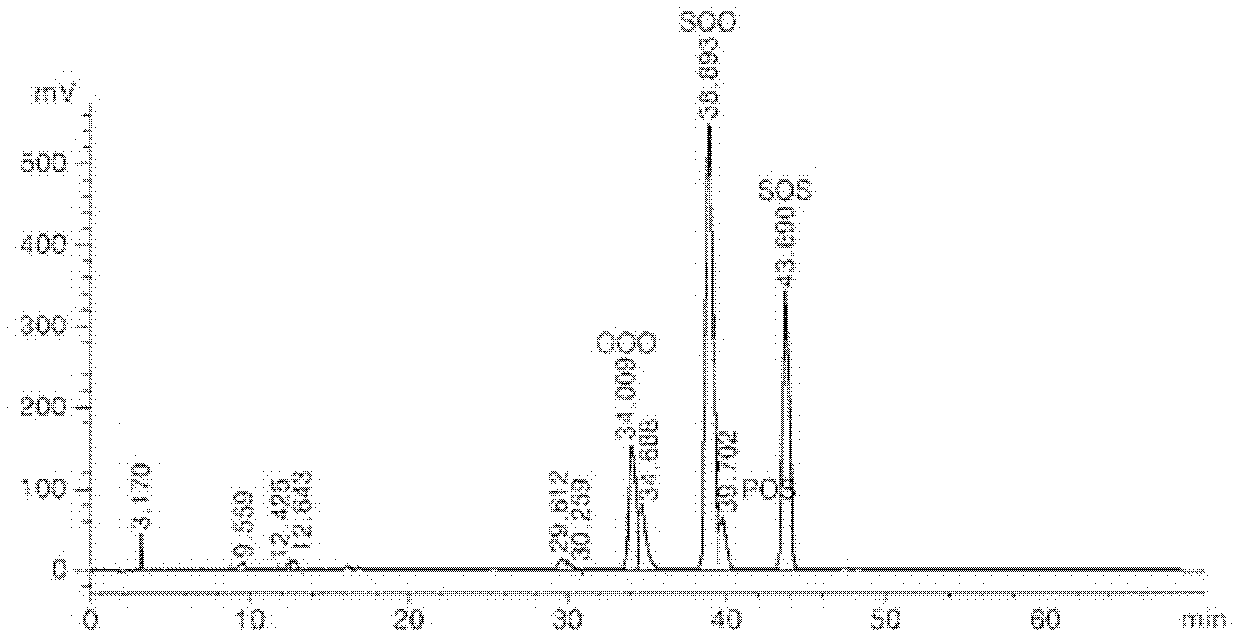
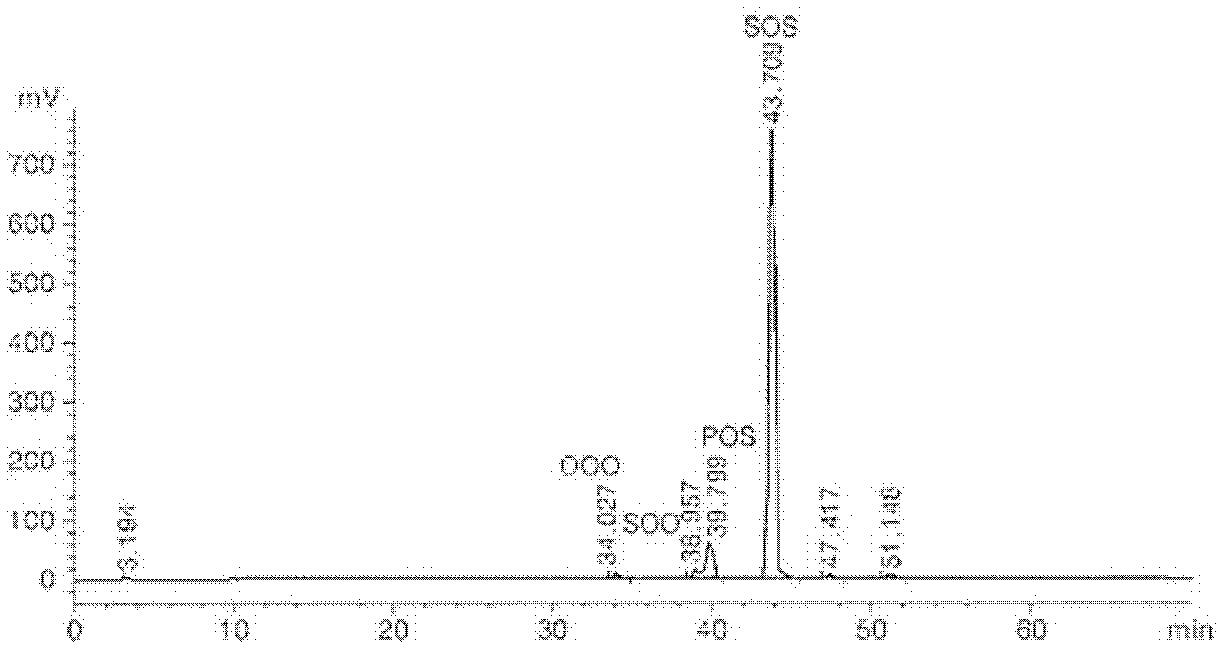
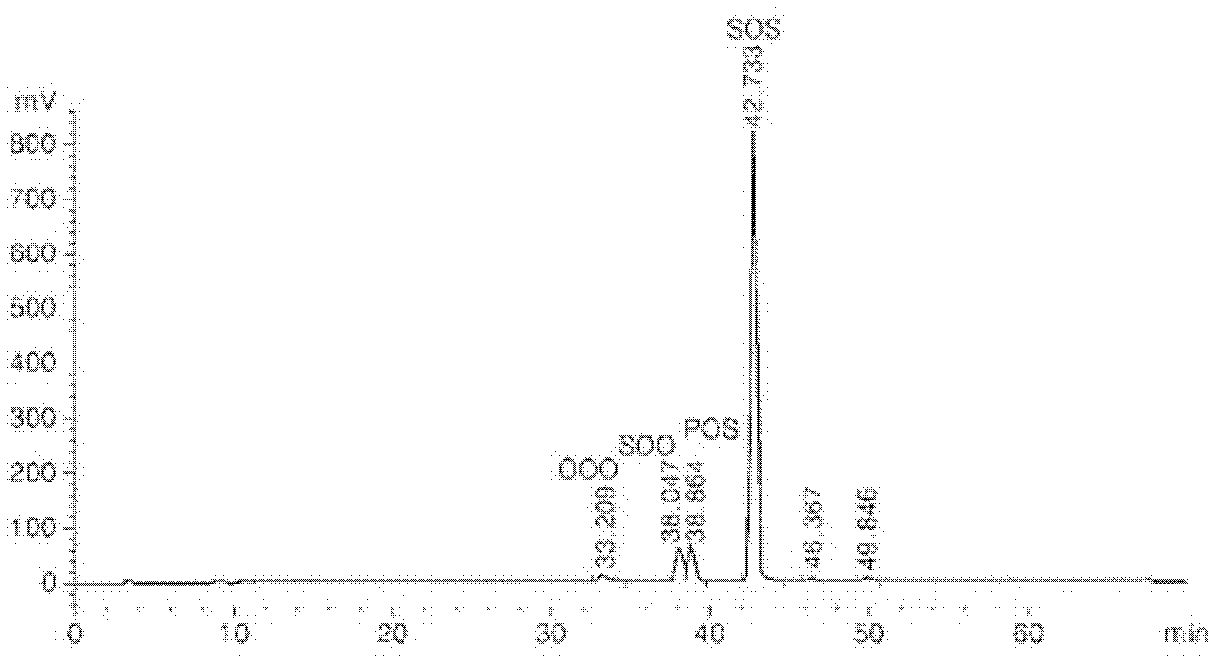
[0043] Experimental example 1: Analysis of the structure of triacylglycerol
[0044] The structure of TAG is changed via enzymatic transesterification, which determines the physical and chemical properties of fats. Therefore, HPLC was used to identify the type and content of TAG in fat before and after enzymatic transesterification in Example 1.
[0045] TAG analysis was performed by HPLC under the conditions listed in Table 1. The TAG structure of fat before and after partial separation was analyzed by reversed-phase high-performance liquid chromatography and evaporative light scattering detector (ELSD). 30 μl of the sample and 10 ml of hexane were filtered using a PEFE syringe filter (25 mm, 0.2 μm), then placed in a 2 mm vial, and a 20 μl sample was injected using an autosampler. Acetonitrile (solvent A) and hexane / isopropanol (solvent B) were used as solvents with a flow rate of 1 ml / min. Solvent gradient elution (A:B, v:v) was performed for a total of 70 min, with 80:2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com