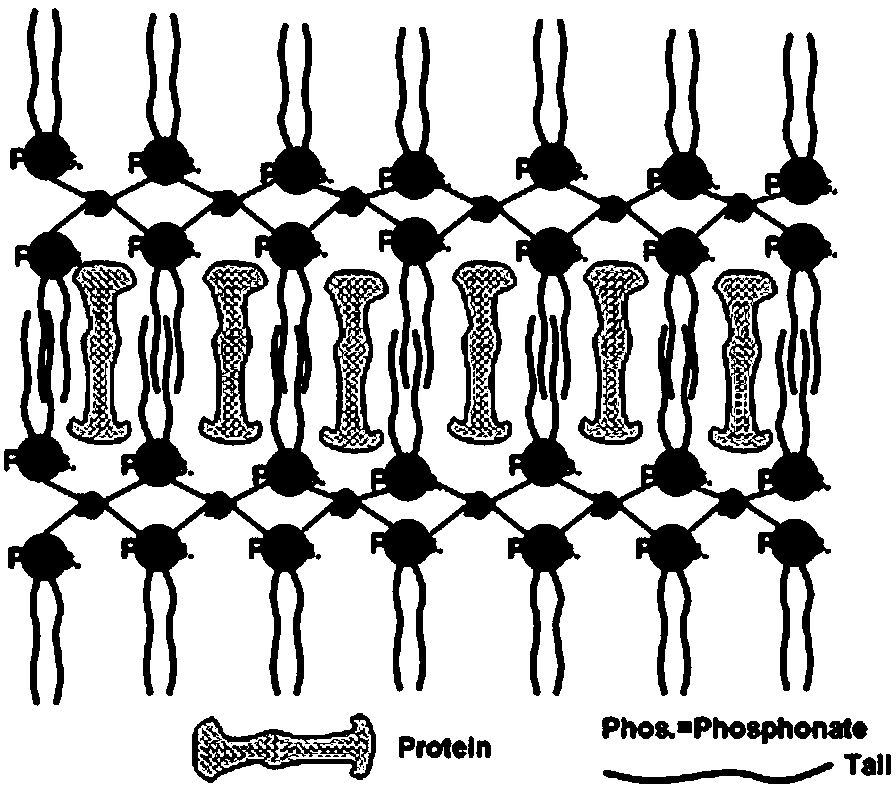
Preparation method of artificial liposome
A liposome and artificial technology, which is applied in the field of pharmacy, can solve the problems of long process cycle, harsh conditions, complicated preparation process of liposome products, etc., and achieve the advantages of simple separation process, mild conditions, and prevention of configuration changes and activity decline. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
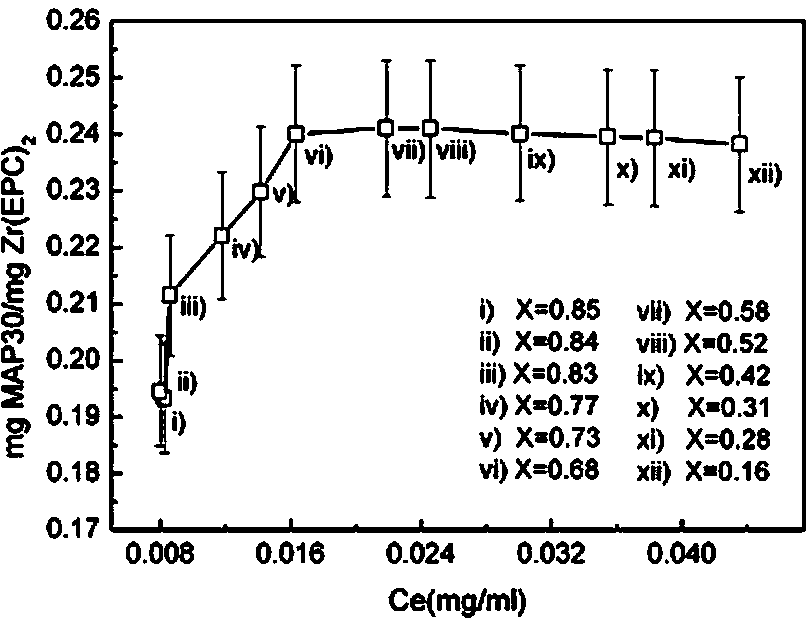
[0029] Preparation of bitter gourd protein MAP30 and egg yolk lecithin zirconium Zr (EPC) 2 Composite liposomes: Momordica charantia protein (from seeds) crude extract, confirmed by SDS-PAGE gel electrophoresis with a molecular weight of 30 kDa, accurately weighed 6.8 mg MAP30 and dissolved in 1.0 ml phosphate buffer (pH 7.2, 10 mM + 100 mM NaCl) for future use; egg yolk lecithin zirconium nanocarriers (5nm, spherical particles) were prepared according to the method in the patent (CN102107131A). Accurately weigh 201.6 μg of Zr(EPC)2 particle carrier, add 1.49 ml of phosphate buffer (pH 7.2), stir at a high speed (3500 rpm) at 4 oC until the carrier is completely suspended and dispersed, then add 0.01 ml of MAP30 protein stock solution , stirred for 0.5, 1.0, 4.0, 7.0, 10.0, 12.0, 15.0, 18.0, 20.0 and 24.0 hours respectively, and separated the supernatant after high-speed centrifugation (12000 rpm) for 20 minutes. The solid particles were washed with 0.1ml phosphate buffer sol...
example 1
[0032] example 1 Artificial liposome products
[0033] The product was freeze-dried for 3 days: white powder, insoluble in water and polar organic solvents; the adsorption time corresponding to the maximum protein content was 15 hours, the optimum pH was 8.52, and the maximum percentage of protein was 24.0 ± 1.0%; After dispersing in phosphate buffer solution, a colorless transparent suspension is obtained; under solid phase conditions, the thermal desorption temperature of protein is 80 oC (within 5 hours); under simulated physiological conditions in vitro, liposomes are stable (protein desorption rate ≤ 5%) for up to 9 months; it is used for the construction and development of stable dosage forms of ribosome-inactivating high-efficiency anticancer drugs, which can be used for the treatment of type I HIV.
[0034] figure 2 Zirconium in egg yolk lecithin (Zr(EPC) for bitter melon protein drug MAP30 2 ) Experimental results of adsorption kinetics of nanocarriers; the experi...
Embodiment 2
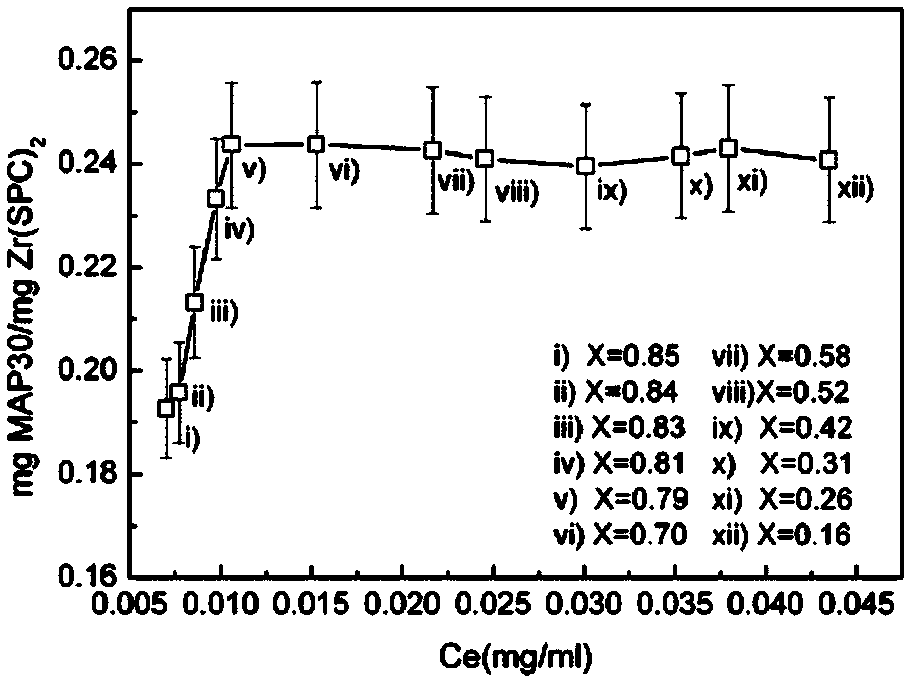
[0036] Preparation of balsam pear protein MAP30 and soybean lecithin zirconium Zr (SPC) 2 Composite liposomes: Accurately weigh 6.8 mg of bitter melon protein, dissolve in 1.0 ml phosphate buffer (pH 7.2, 10 mM + 100 mM NaCl) and save for later use; soybean lecithin zirconium nanocarriers (5nm, spherical particles) are patented (CN102107131A) prepared by the method. Accurately weigh 201.6 μg Zr(SPC) 2 For particle carrier, add 1.49 ml phosphate buffer (pH 7.2), stir at high speed (3500 rpm) at 4 oC until the carrier is completely suspended and dispersed, then add 0.01 ml MAP30 protein stock solution, stir 0.5, 1.0, 4.0, 7.0 respectively , 10.0, 12.0, 15.0, 18.0, 20.0 and 24.0 hours, after high-speed centrifugation (12000 rpm) for 20 minutes, the supernatant was separated. The solid particles were washed with 0.1ml phosphate buffer solution and then centrifuged, repeated 3 times, the supernatant was combined to measure the residual protein amount, and the optimal adsorption t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com